Huy-Dung Nguyen
Enhancing Steering Estimation with Semantic-Aware GNNs
Mar 21, 2025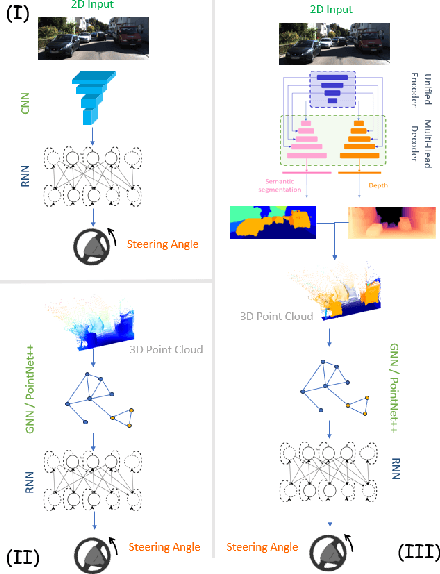
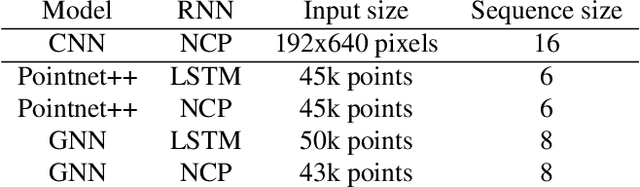
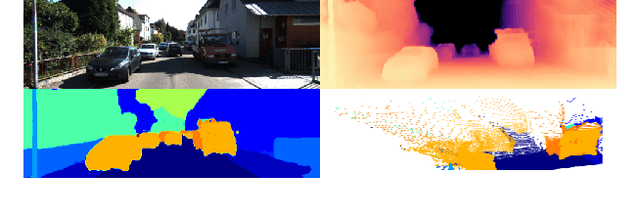
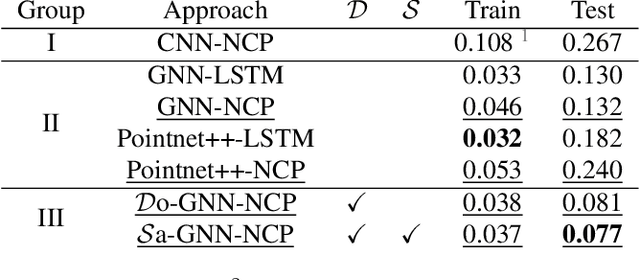
Abstract:Steering estimation is a critical task in autonomous driving, traditionally relying on 2D image-based models. In this work, we explore the advantages of incorporating 3D spatial information through hybrid architectures that combine 3D neural network models with recurrent neural networks (RNNs) for temporal modeling, using LiDAR-based point clouds as input. We systematically evaluate four hybrid 3D models, all of which outperform the 2D-only baseline, with the Graph Neural Network (GNN) - RNN model yielding the best results. To reduce reliance on LiDAR, we leverage a pretrained unified model to estimate depth from monocular images, reconstructing pseudo-3D point clouds. We then adapt the GNN-RNN model, originally designed for LiDAR-based point clouds, to work with these pseudo-3D representations, achieving comparable or even superior performance compared to the LiDAR-based model. Additionally, the unified model provides semantic labels for each point, enabling a more structured scene representation. To further optimize graph construction, we introduce an efficient connectivity strategy where connections are predominantly formed between points of the same semantic class, with only 20\% of inter-class connections retained. This targeted approach reduces graph complexity and computational cost while preserving critical spatial relationships. Finally, we validate our approach on the KITTI dataset, achieving a 71% improvement over 2D-only models. Our findings highlight the advantages of 3D spatial information and efficient graph construction for steering estimation, while maintaining the cost-effectiveness of monocular images and avoiding the expense of LiDAR-based systems.
Human Insights Driven Latent Space for Different Driving Perspectives: A Unified Encoder for Efficient Multi-Task Inference
Sep 16, 2024



Abstract:Autonomous driving holds great potential to transform road safety and traffic efficiency by minimizing human error and reducing congestion. A key challenge in realizing this potential is the accurate estimation of steering angles, which is essential for effective vehicle navigation and control. Recent breakthroughs in deep learning have made it possible to estimate steering angles directly from raw camera inputs. However, the limited available navigation data can hinder optimal feature learning, impacting the system's performance in complex driving scenarios. In this paper, we propose a shared encoder trained on multiple computer vision tasks critical for urban navigation, such as depth, pose, and 3D scene flow estimation, as well as semantic, instance, panoptic, and motion segmentation. By incorporating diverse visual information used by humans during navigation, this unified encoder might enhance steering angle estimation. To achieve effective multi-task learning within a single encoder, we introduce a multi-scale feature network for pose estimation to improve depth learning. Additionally, we employ knowledge distillation from a multi-backbone model pretrained on these navigation tasks to stabilize training and boost performance. Our findings demonstrate that a shared backbone trained on diverse visual tasks is capable of providing overall perception capabilities. While our performance in steering angle estimation is comparable to existing methods, the integration of human-like perception through multi-task learning holds significant potential for advancing autonomous driving systems. More details and the pretrained model are available at https://hi-computervision.github.io/uni-encoder/.
3D Transformer based on deformable patch location for differential diagnosis between Alzheimer's disease and Frontotemporal dementia
Sep 06, 2023



Abstract:Alzheimer's disease and Frontotemporal dementia are common types of neurodegenerative disorders that present overlapping clinical symptoms, making their differential diagnosis very challenging. Numerous efforts have been done for the diagnosis of each disease but the problem of multi-class differential diagnosis has not been actively explored. In recent years, transformer-based models have demonstrated remarkable success in various computer vision tasks. However, their use in disease diagnostic is uncommon due to the limited amount of 3D medical data given the large size of such models. In this paper, we present a novel 3D transformer-based architecture using a deformable patch location module to improve the differential diagnosis of Alzheimer's disease and Frontotemporal dementia. Moreover, to overcome the problem of data scarcity, we propose an efficient combination of various data augmentation techniques, adapted for training transformer-based models on 3D structural magnetic resonance imaging data. Finally, we propose to combine our transformer-based model with a traditional machine learning model using brain structure volumes to better exploit the available data. Our experiments demonstrate the effectiveness of the proposed approach, showing competitive results compared to state-of-the-art methods. Moreover, the deformable patch locations can be visualized, revealing the most relevant brain regions used to establish the diagnosis of each disease.
Brain Structure Ages -- A new biomarker for multi-disease classification
Apr 13, 2023Abstract:Age is an important variable to describe the expected brain's anatomy status across the normal aging trajectory. The deviation from that normative aging trajectory may provide some insights into neurological diseases. In neuroimaging, predicted brain age is widely used to analyze different diseases. However, using only the brain age gap information (\ie the difference between the chronological age and the estimated age) can be not enough informative for disease classification problems. In this paper, we propose to extend the notion of global brain age by estimating brain structure ages using structural magnetic resonance imaging. To this end, an ensemble of deep learning models is first used to estimate a 3D aging map (\ie voxel-wise age estimation). Then, a 3D segmentation mask is used to obtain the final brain structure ages. This biomarker can be used in several situations. First, it enables to accurately estimate the brain age for the purpose of anomaly detection at the population level. In this situation, our approach outperforms several state-of-the-art methods. Second, brain structure ages can be used to compute the deviation from the normal aging process of each brain structure. This feature can be used in a multi-disease classification task for an accurate differential diagnosis at the subject level. Finally, the brain structure age deviations of individuals can be visualized, providing some insights about brain abnormality and helping clinicians in real medical contexts.
Deep Grading based on Collective Artificial Intelligence for AD Diagnosis and Prognosis
Nov 28, 2022Abstract:Accurate diagnosis and prognosis of Alzheimer's disease are crucial to develop new therapies and reduce the associated costs. Recently, with the advances of convolutional neural networks, methods have been proposed to automate these two tasks using structural MRI. However, these methods often suffer from lack of interpretability, generalization, and can be limited in terms of performance. In this paper, we propose a novel deep framework designed to overcome these limitations. Our framework consists of two stages. In the first stage, we propose a deep grading model to extract meaningful features. To enhance the robustness of these features against domain shift, we introduce an innovative collective artificial intelligence strategy for training and evaluating steps. In the second stage, we use a graph convolutional neural network to better capture AD signatures. Our experiments based on 2074 subjects show the competitive performance of our deep framework compared to state-of-the-art methods on different datasets for both AD diagnosis and prognosis.
Deep grading for MRI-based differential diagnosis of Alzheimer's disease and Frontotemporal dementia
Nov 25, 2022Abstract:Alzheimer's disease and Frontotemporal dementia are common forms of neurodegenerative dementia. Behavioral alterations and cognitive impairments are found in the clinical courses of both diseases and their differential diagnosis is sometimes difficult for physicians. Therefore, an accurate tool dedicated to this diagnostic challenge can be valuable in clinical practice. However, current structural imaging methods mainly focus on the detection of each disease but rarely on their differential diagnosis. In this paper, we propose a deep learning based approach for both problems of disease detection and differential diagnosis. We suggest utilizing two types of biomarkers for this application: structure grading and structure atrophy. First, we propose to train a large ensemble of 3D U-Nets to locally determine the anatomical patterns of healthy people, patients with Alzheimer's disease and patients with Frontotemporal dementia using structural MRI as input. The output of the ensemble is a 2-channel disease's coordinate map able to be transformed into a 3D grading map which is easy to interpret for clinicians. This 2-channel map is coupled with a multi-layer perceptron classifier for different classification tasks. Second, we propose to combine our deep learning framework with a traditional machine learning strategy based on volume to improve the model discriminative capacity and robustness. After both cross-validation and external validation, our experiments based on 3319 MRI demonstrated competitive results of our method compared to the state-of-the-art methods for both disease detection and differential diagnosis.
Interpretable differential diagnosis for Alzheimer's disease and Frontotemporal dementia
Jun 15, 2022
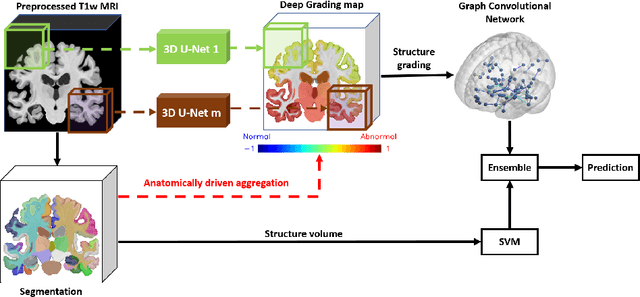
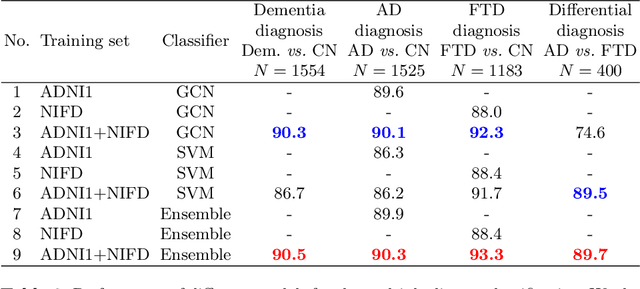
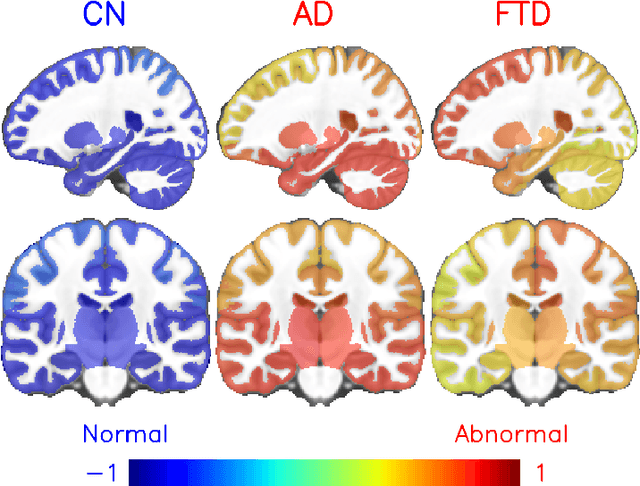
Abstract:Alzheimer's disease and Frontotemporal dementia are two major types of dementia. Their accurate diagnosis and differentiation is crucial for determining specific intervention and treatment. However, differential diagnosis of these two types of dementia remains difficult at the early stage of disease due to similar patterns of clinical symptoms. Therefore, the automatic classification of multiple types of dementia has an important clinical value. So far, this challenge has not been actively explored. Recent development of deep learning in the field of medical image has demonstrated high performance for various classification tasks. In this paper, we propose to take advantage of two types of biomarkers: structure grading and structure atrophy. To this end, we propose first to train a large ensemble of 3D U-Nets to locally discriminate healthy versus dementia anatomical patterns. The result of these models is an interpretable 3D grading map capable of indicating abnormal brain regions. This map can also be exploited in various classification tasks using graph convolutional neural network. Finally, we propose to combine deep grading and atrophy-based classifications to improve dementia type discrimination. The proposed framework showed competitive performance compared to state-of-the-art methods for different tasks of disease detection and differential diagnosis.
Towards better Interpretable and Generalizable AD detection using Collective Artificial Intelligence
Jun 07, 2022

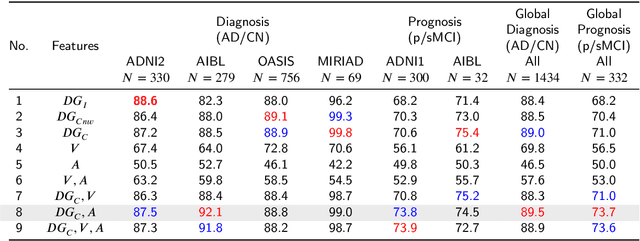

Abstract:Accurate diagnosis and prognosis of Alzheimer's disease are crucial for developing new therapies and reducing the associated costs. Recently, with the advances of convolutional neural networks, deep learning methods have been proposed to automate these two tasks using structural MRI. However, these methods often suffer from a lack of interpretability and generalization and have limited prognosis performance. In this paper, we propose a novel deep framework designed to overcome these limitations. Our pipeline consists of two stages. In the first stage, 125 3D U-Nets are used to estimate voxelwise grade scores over the whole brain. The resulting 3D maps are then fused to construct an interpretable 3D grading map indicating the disease severity at the structure level. As a consequence, clinicians can use this map to detect the brain structures affected by the disease. In the second stage, the grading map and subject's age are used to perform classification with a graph convolutional neural network. Experimental results based on 2106 subjects demonstrated competitive performance of our deep framework compared to state-of-the-art methods on different datasets for both AD diagnosis and prognosis. Moreover, we found that using a large number of U-Nets processing different overlapping brain areas improved the generalization capacity of the proposed methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge