Hongbing Lu
Multi-region segmentation of bladder cancer structures in MRI with progressive dilated convolutional networks
Aug 09, 2018



Abstract:Precise segmentation of bladder walls and tumor regions is an essential step towards non-invasive identification of tumor stage and grade, which is critical for treatment decision and prognosis of patients with bladder cancer (BC). However, the automatic delineation of bladder walls and tumor in magnetic resonance images (MRI) is a challenging task, due to important bladder shape variations, strong intensity inhomogeneity in urine and very high variability across population, particularly on tumors appearance. To tackle these issues, we propose to use a deep fully convolutional neural network. The proposed network includes dilated convolutions to increase the receptive field without incurring extra cost nor degrading its performance. Furthermore, we introduce progressive dilations in each convolutional block, thereby enabling extensive receptive fields without the need for large dilation rates. The proposed network is evaluated on 3.0T T2-weighted MRI scans from 60 pathologically confirmed patients with BC. Experiments shows the proposed model to achieve high accuracy, with a mean Dice similarity coefficient of 0.98, 0.84 and 0.69 for inner wall, outer wall and tumor region, respectively. These results represent a very good agreement with reference contours and an increase in performance compared to existing methods. In addition, inference times are less than a second for a whole 3D volume, which is between 2-3 orders of magnitude faster than related state-of-the-art methods for this application. We showed that a CNN can yield precise segmentation of bladder walls and tumors in bladder cancer patients on MRI. The whole segmentation process is fully-automatic and yields results in very good agreement with the reference standard, demonstrating the viability of deep learning models for the automatic multi-region segmentation of bladder cancer MRI images.
Statistical models and regularization strategies in statistical image reconstruction of low-dose X-ray CT: a survey
May 14, 2015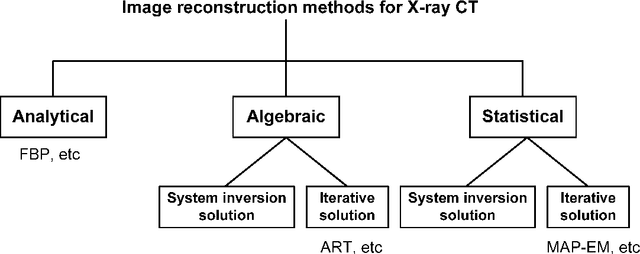
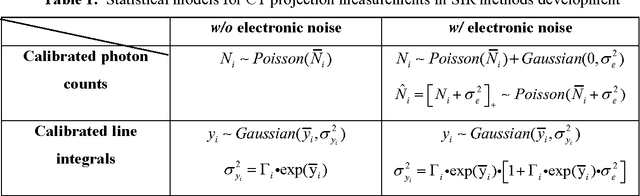
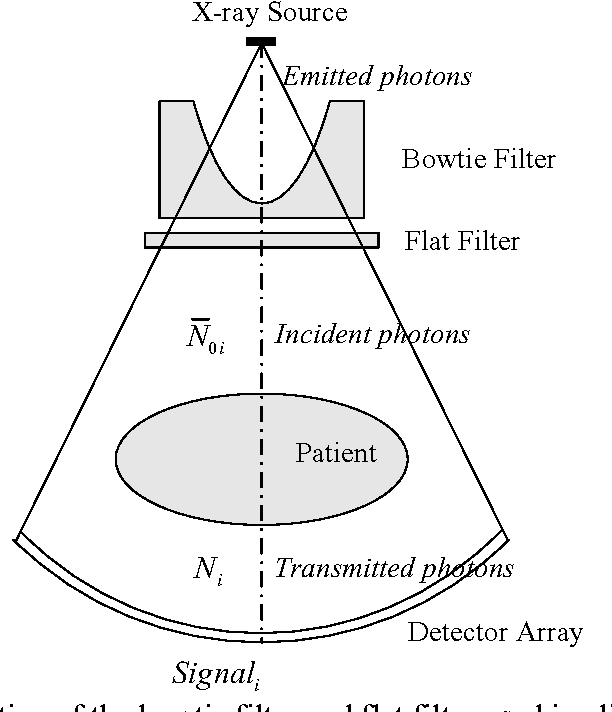
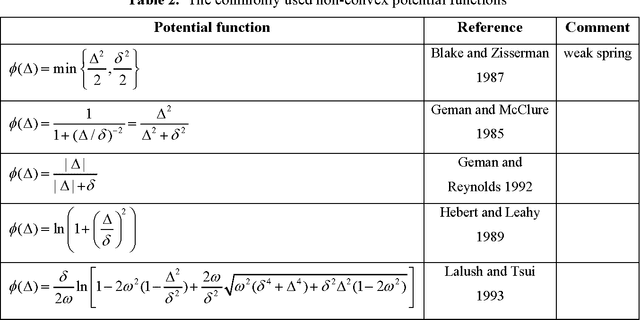
Abstract:Statistical image reconstruction (SIR) methods have shown potential to substantially improve the image quality of low-dose X-ray computed tomography (CT) as compared to the conventional filtered back-projection (FBP) method for various clinical tasks. According to the maximum a posterior (MAP) estimation, the SIR methods can be typically formulated by an objective function consisting of two terms: (1) data-fidelity (or equivalently, data-fitting or data-mismatch) term modeling the statistics of projection measurements, and (2) regularization (or equivalently, prior or penalty) term reflecting prior knowledge or expectation on the characteristics of the image to be reconstructed. Existing SIR methods for low-dose CT can be divided into two groups: (1) those that use calibrated transmitted photon counts (before log-transform) with penalized maximum likelihood (pML) criterion, and (2) those that use calibrated line-integrals (after log-transform) with penalized weighted least-squares (PWLS) criterion. Accurate statistical modeling of the projection measurements is a prerequisite for SIR, while the regularization term in the objective function also plays a critical role for successful image reconstruction. This paper reviews several statistical models on CT projection measurements and various regularization strategies incorporating prior knowledge or expected properties of the image to be reconstructed, which together formulate the objective function of the SIR methods for low-dose X-ray CT.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge