Hongbin Han
QSMnet-INR: Single-Orientation Quantitative Susceptibility Mapping via Implicit Neural Representation in k-Space
Dec 10, 2025Abstract:Quantitative Susceptibility Mapping (QSM) quantifies tissue magnetic susceptibility from magnetic-resonance phase data and plays a crucial role in brain microstructure imaging, iron-deposition assessment, and neurological-disease research. However, single-orientation QSM inversion remains highly ill-posed because the dipole kernel exhibits a cone-null region in the Fourier domain, leading to streaking artifacts and structural loss. To overcome this limitation, we propose QSMnet-INR, a deep, physics-informed framework that integrates an Implicit Neural Representation (INR) into the k-space domain. The INR module continuously models multi-directional dipole responses and explicitly completes the cone-null region, while a frequency-domain residual-weighted Dipole Loss enforces physical consistency. The overall network combines a 3D U-Net-based QSMnet backbone with the INR module through alternating optimization for end-to-end joint training. Experiments on the 2016 QSM Reconstruction Challenge, a multi-orientation GRE dataset, and both in-house and public single-orientation clinical data demonstrate that QSMnet-INR consistently outperforms conventional and recent deep-learning approaches across multiple quantitative metrics. The proposed framework shows notable advantages in structural recovery within cone-null regions and in artifact suppression. Ablation studies further confirm the complementary contributions of the INR module and Dipole Loss to detail preservation and physical stability. Overall, QSMnet-INR effectively alleviates the ill-posedness of single-orientation QSM without requiring multi-orientation acquisition, achieving high accuracy, robustness, and strong cross-scenario generalization-highlighting its potential for clinical translation.
Modality-Projection Universal Model for Comprehensive Full-Body Medical Imaging Segmentation
Dec 26, 2024Abstract:The integration of deep learning in medical imaging has shown great promise for enhancing diagnostic, therapeutic, and research outcomes. However, applying universal models across multiple modalities remains challenging due to the inherent variability in data characteristics. This study aims to introduce and evaluate a Modality Projection Universal Model (MPUM). MPUM employs a novel modality-projection strategy, which allows the model to dynamically adjust its parameters to optimize performance across different imaging modalities. The MPUM demonstrated superior accuracy in identifying anatomical structures, enabling precise quantification for improved clinical decision-making. It also identifies metabolic associations within the brain-body axis, advancing research on brain-body physiological correlations. Furthermore, MPUM's unique controller-based convolution layer enables visualization of saliency maps across all network layers, significantly enhancing the model's interpretability.
Quantitative Analysis of Molecular Transport in the Extracellular Space Using Physics-Informed Neural Network
Jan 24, 2024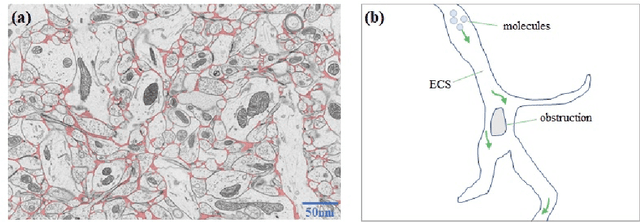



Abstract:The brain extracellular space (ECS), an irregular, extremely tortuous nanoscale space located between cells or between cells and blood vessels, is crucial for nerve cell survival. It plays a pivotal role in high-level brain functions such as memory, emotion, and sensation. However, the specific form of molecular transport within the ECS remain elusive. To address this challenge, this paper proposes a novel approach to quantitatively analyze the molecular transport within the ECS by solving an inverse problem derived from the advection-diffusion equation (ADE) using a physics-informed neural network (PINN). PINN provides a streamlined solution to the ADE without the need for intricate mathematical formulations or grid settings. Additionally, the optimization of PINN facilitates the automatic computation of the diffusion coefficient governing long-term molecule transport and the velocity of molecules driven by advection. Consequently, the proposed method allows for the quantitative analysis and identification of the specific pattern of molecular transport within the ECS through the calculation of the Peclet number. Experimental validation on two datasets of magnetic resonance images (MRIs) captured at different time points showcases the effectiveness of the proposed method. Notably, our simulations reveal identical molecular transport patterns between datasets representing rats with tracer injected into the same brain region. These findings highlight the potential of PINN as a promising tool for comprehensively exploring molecular transport within the ECS.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge