Haotian Zhao
Kimi K2: Open Agentic Intelligence
Jul 28, 2025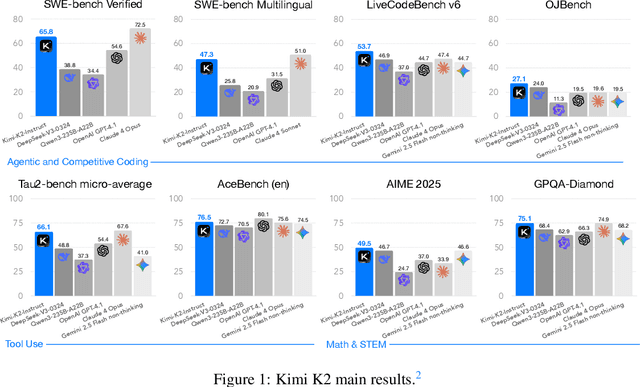
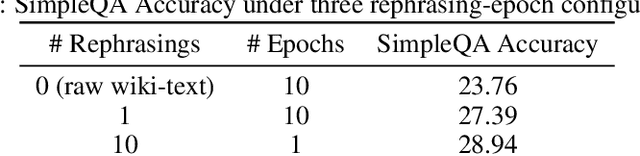
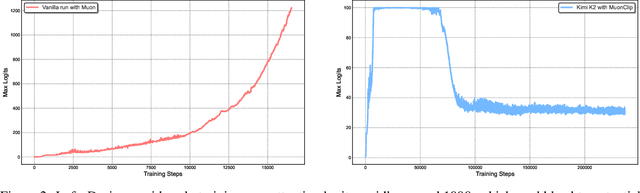
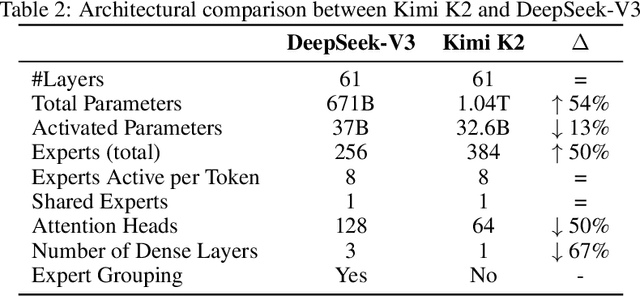
Abstract:We introduce Kimi K2, a Mixture-of-Experts (MoE) large language model with 32 billion activated parameters and 1 trillion total parameters. We propose the MuonClip optimizer, which improves upon Muon with a novel QK-clip technique to address training instability while enjoying the advanced token efficiency of Muon. Based on MuonClip, K2 was pre-trained on 15.5 trillion tokens with zero loss spike. During post-training, K2 undergoes a multi-stage post-training process, highlighted by a large-scale agentic data synthesis pipeline and a joint reinforcement learning (RL) stage, where the model improves its capabilities through interactions with real and synthetic environments. Kimi K2 achieves state-of-the-art performance among open-source non-thinking models, with strengths in agentic capabilities. Notably, K2 obtains 66.1 on Tau2-Bench, 76.5 on ACEBench (En), 65.8 on SWE-Bench Verified, and 47.3 on SWE-Bench Multilingual -- surpassing most open and closed-sourced baselines in non-thinking settings. It also exhibits strong capabilities in coding, mathematics, and reasoning tasks, with a score of 53.7 on LiveCodeBench v6, 49.5 on AIME 2025, 75.1 on GPQA-Diamond, and 27.1 on OJBench, all without extended thinking. These results position Kimi K2 as one of the most capable open-source large language models to date, particularly in software engineering and agentic tasks. We release our base and post-trained model checkpoints to facilitate future research and applications of agentic intelligence.
Reconstructing Quantitative Cerebral Perfusion Images Directly From Measured Sinogram Data Acquired Using C-arm Cone-Beam CT
Dec 06, 2024



Abstract:To shorten the door-to-puncture time for better treating patients with acute ischemic stroke, it is highly desired to obtain quantitative cerebral perfusion images using C-arm cone-beam computed tomography (CBCT) equipped in the interventional suite. However, limited by the slow gantry rotation speed, the temporal resolution and temporal sampling density of typical C-arm CBCT are much poorer than those of multi-detector-row CT in the diagnostic imaging suite. The current quantitative perfusion imaging includes two cascaded steps: time-resolved image reconstruction and perfusion parametric estimation. For time-resolved image reconstruction, the technical challenge imposed by poor temporal resolution and poor sampling density causes inaccurate quantification of the temporal variation of cerebral artery and tissue attenuation values. For perfusion parametric estimation, it remains a technical challenge to appropriately design the handcrafted regularization for better solving the associated deconvolution problem. These two challenges together prevent obtaining quantitatively accurate perfusion images using C-arm CBCT. The purpose of this work is to simultaneously address these two challenges by combining the two cascaded steps into a single joint optimization problem and reconstructing quantitative perfusion images directly from the measured sinogram data. In the developed direct cerebral perfusion parametric image reconstruction technique, TRAINER in short, the quantitative perfusion images have been represented as a subject-specific conditional generative model trained under the constraint of the time-resolved CT forward model, perfusion convolutional model, and the subject's own measured sinogram data. Results shown in this paper demonstrated that using TRAINER, quantitative cerebral perfusion images can be accurately obtained using C-arm CBCT in the interventional suite.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge