Hairong Shi
VideoEspresso: A Large-Scale Chain-of-Thought Dataset for Fine-Grained Video Reasoning via Core Frame Selection
Nov 22, 2024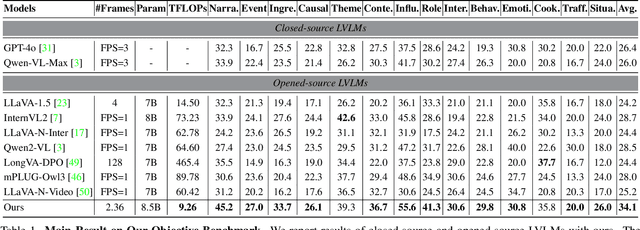
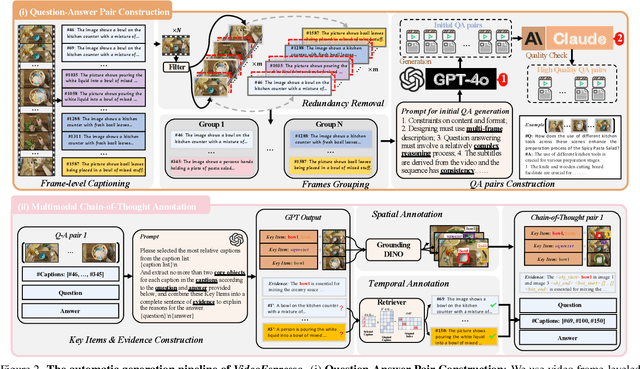
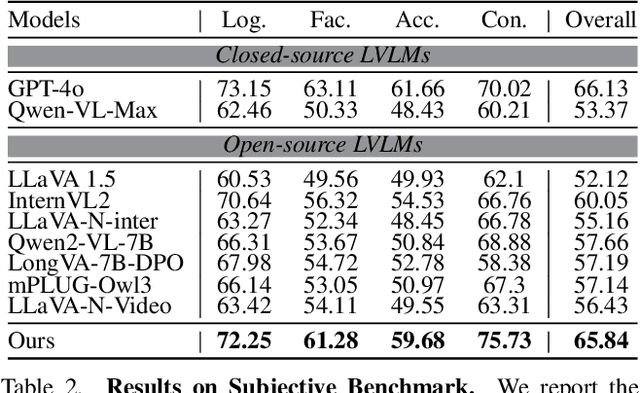
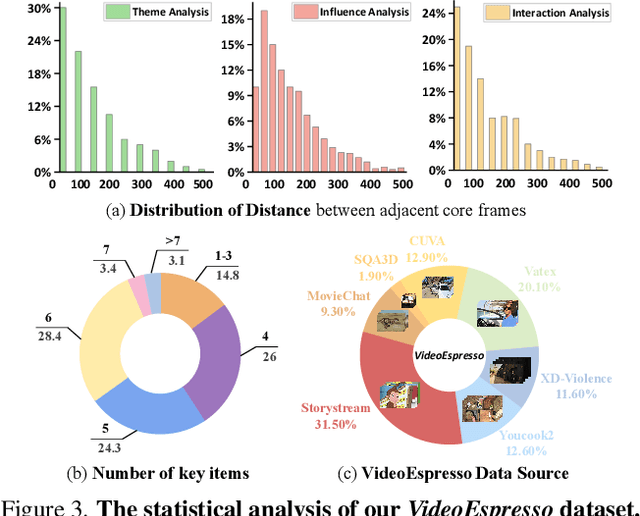
Abstract:The advancement of Large Vision Language Models (LVLMs) has significantly improved multimodal understanding, yet challenges remain in video reasoning tasks due to the scarcity of high-quality, large-scale datasets. Existing video question-answering (VideoQA) datasets often rely on costly manual annotations with insufficient granularity or automatic construction methods with redundant frame-by-frame analysis, limiting their scalability and effectiveness for complex reasoning. To address these challenges, we introduce VideoEspresso, a novel dataset that features VideoQA pairs preserving essential spatial details and temporal coherence, along with multimodal annotations of intermediate reasoning steps. Our construction pipeline employs a semantic-aware method to reduce redundancy, followed by generating QA pairs using GPT-4o. We further develop video Chain-of-Thought (CoT) annotations to enrich reasoning processes, guiding GPT-4o in extracting logical relationships from QA pairs and video content. To exploit the potential of high-quality VideoQA pairs, we propose a Hybrid LVLMs Collaboration framework, featuring a Frame Selector and a two-stage instruction fine-tuned reasoning LVLM. This framework adaptively selects core frames and performs CoT reasoning using multimodal evidence. Evaluated on our proposed benchmark with 14 tasks against 9 popular LVLMs, our method outperforms existing baselines on most tasks, demonstrating superior video reasoning capabilities. Our code and dataset will be released at: https://github.com/hshjerry/VideoEspresso
Mask-Enhanced Segment Anything Model for Tumor Lesion Semantic Segmentation
Mar 09, 2024Abstract:Tumor lesion segmentation on CT or MRI images plays a critical role in cancer diagnosis and treatment planning. Considering the inherent differences in tumor lesion segmentation data across various medical imaging modalities and equipment, integrating medical knowledge into the Segment Anything Model (SAM) presents promising capability due to its versatility and generalization potential. Recent studies have attempted to enhance SAM with medical expertise by pre-training on large-scale medical segmentation datasets. However, challenges still exist in 3D tumor lesion segmentation owing to tumor complexity and the imbalance in foreground and background regions. Therefore, we introduce Mask-Enhanced SAM (M-SAM), an innovative architecture tailored for 3D tumor lesion segmentation. We propose a novel Mask-Enhanced Adapter (MEA) within M-SAM that enriches the semantic information of medical images with positional data from coarse segmentation masks, facilitating the generation of more precise segmentation masks. Furthermore, an iterative refinement scheme is implemented in M-SAM to refine the segmentation masks progressively, leading to improved performance. Extensive experiments on seven tumor lesion segmentation datasets indicate that our M-SAM not only achieves high segmentation accuracy but also exhibits robust generalization.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge