Håkan Ahlström
Section of Radiology, Department of Surgical Sciences, Uppsala University, Uppsala, Sweden, Antaros Medical, Mölndal, Sweden
Towards prediction of morphological heart age from computed tomography angiography
Apr 22, 2025Abstract:Age prediction from medical images or other health-related non-imaging data is an important approach to data-driven aging research, providing knowledge of how much information a specific tissue or organ carries about the chronological age of the individual. In this work, we studied the prediction of age from computed tomography angiography (CTA) images, which provide detailed representations of the heart morphology, with the goals of (i) studying the relationship between morphology and aging, and (ii) developing a novel \emph{morphological heart age} biomarker. We applied an image registration-based method that standardizes the images from the whole cohort into a single space. We then extracted supervoxels (using unsupervised segmentation), and corresponding robust features of density and local volume, which provide a detailed representation of the heart morphology while being robust to registration errors. Machine learning models are then trained to fit regression models from these features to the chronological age. We applied the method to a subset of the images from the Swedish CArdioPulomonary bioImage Study (SCAPIS) dataset, consisting of 721 females and 666 males. We observe a mean absolute error of $2.74$ years for females and $2.77$ years for males. The predictions from different sub-regions of interest were observed to be more highly correlated with the predictions from the whole heart, compared to the chronological age, revealing a high consistency in the predictions from morphology. Saliency analysis was also performed on the prediction models to study what regions are associated positively and negatively with the predicted age. This resulted in detailed association maps where the density and volume of known, as well as some novel sub-regions of interest, are determined to be important. The saliency analysis aids in the interpretability of the models and their predictions.
A method for supervoxel-wise association studies of age and other non-imaging variables from coronary computed tomography angiograms
May 13, 2024Abstract:The study of associations between an individual's age and imaging and non-imaging data is an active research area that attempts to aid understanding of the effects and patterns of aging. In this work we have conducted a supervoxel-wise association study between both volumetric and tissue density features in coronary computed tomography angiograms and the chronological age of a subject, to understand the localized changes in morphology and tissue density with age. To enable a supervoxel-wise study of volume and tissue density, we developed a novel method based on image segmentation, inter-subject image registration, and robust supervoxel-based correlation analysis, to achieve a statistical association study between the images and age. We evaluate the registration methodology in terms of the Dice coefficient for the heart chambers and myocardium, and the inverse consistency of the transformations, showing that the method works well in most cases with high overlap and inverse consistency. In a sex-stratified study conducted on a subset of $n=1388$ images from the SCAPIS study, the supervoxel-wise analysis was able to find localized associations with age outside of the commonly segmented and analyzed sub-regions, and several substantial differences between the sexes in association of age and volume.
Leveraging point annotations in segmentation learning with boundary loss
Nov 06, 2023Abstract:This paper investigates the combination of intensity-based distance maps with boundary loss for point-supervised semantic segmentation. By design the boundary loss imposes a stronger penalty on the false positives the farther away from the object they occur. Hence it is intuitively inappropriate for weak supervision, where the ground truth label may be much smaller than the actual object and a certain amount of false positives (w.r.t. the weak ground truth) is actually desirable. Using intensity-aware distances instead may alleviate this drawback, allowing for a certain amount of false positives without a significant increase to the training loss. The motivation for applying the boundary loss directly under weak supervision lies in its great success for fully supervised segmentation tasks, but also in not requiring extra priors or outside information that is usually required -- in some form -- with existing weakly supervised methods in the literature. This formulation also remains potentially more attractive than existing CRF-based regularizers, due to its simplicity and computational efficiency. We perform experiments on two multi-class datasets; ACDC (heart segmentation) and POEM (whole-body abdominal organ segmentation). Preliminary results are encouraging and show that this supervision strategy has great potential. On ACDC it outperforms the CRF-loss based approach, and on POEM data it performs on par with it. The code for all our experiments is openly available.
MIMIR: Deep Regression for Automated Analysis of UK Biobank Body MRI
Jun 22, 2021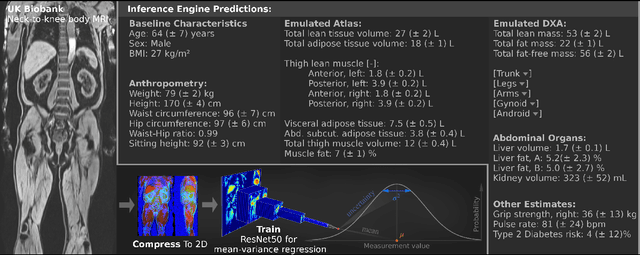
Abstract:UK Biobank (UKB) is conducting a large-scale study of more than half a million volunteers, collecting health-related information on genetics, lifestyle, blood biochemistry, and more. Medical imaging furthermore targets 100,000 subjects, with 70,000 follow-up sessions, enabling measurements of organs, muscle, and body composition. With up to 170,000 mounting MR images, various methodologies are accordingly engaged in large-scale image analysis. This work presents an experimental inference engine that can automatically predict a comprehensive profile of subject metadata from UKB neck-to-knee body MRI. In cross-validation, it accurately inferred baseline characteristics such as age, height, weight, and sex, but also emulated measurements of body composition by DXA, organ volumes, and abstract properties like grip strength, pulse rate, and type 2 diabetic status (AUC: 0.866). The proposed system can automatically analyze thousands of subjects within hours and provide individual confidence intervals. The underlying methodology is based on convolutional neural networks for image-based mean-variance regression on two-dimensional representations of the MRI data. This work aims to make the proposed system available for free to researchers, who can use it to obtain fast and fully-automated estimates of 72 different measurements immediately upon release of new UK Biobank image data.
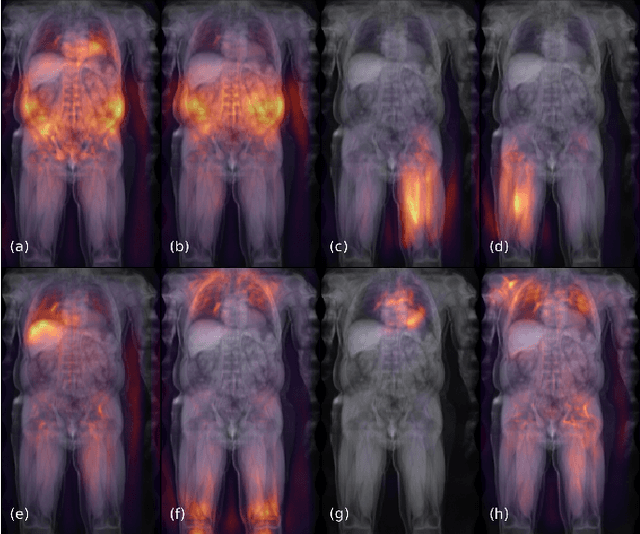
Deep regression for uncertainty-aware and interpretable analysis of large-scale body MRI
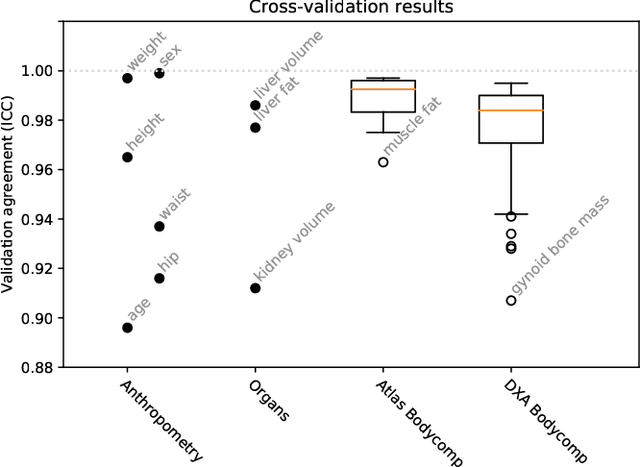
May 17, 2021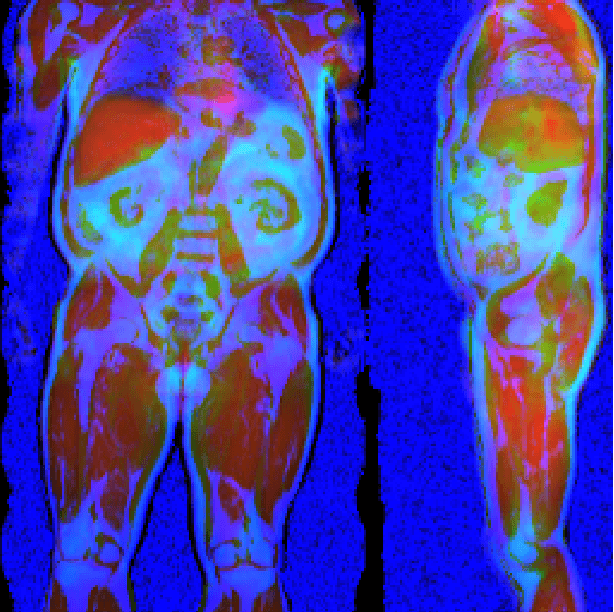
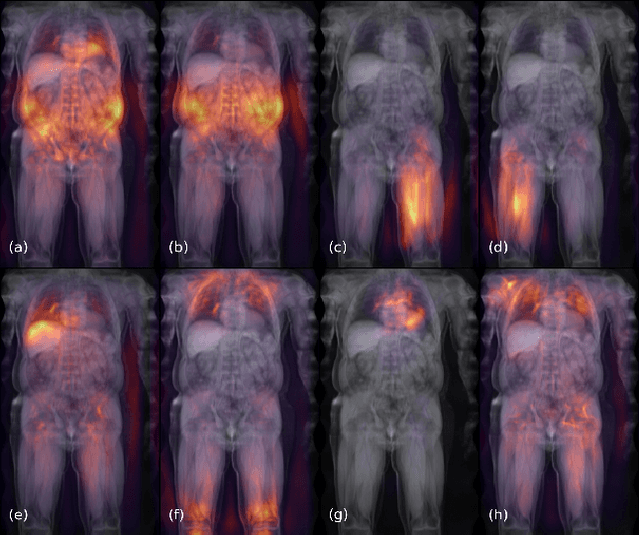
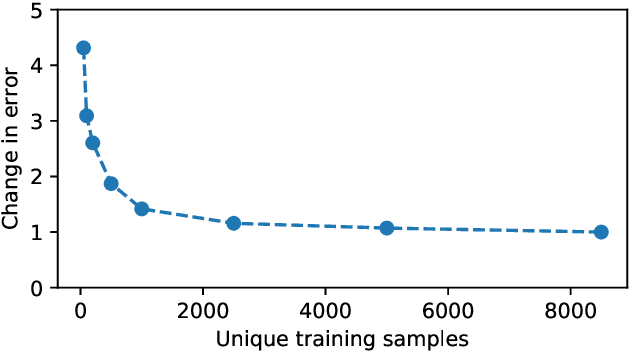
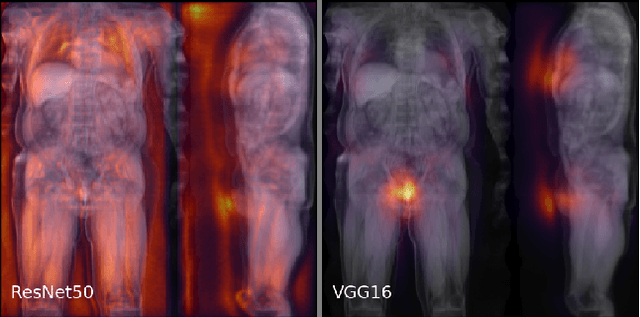
Abstract:Large-scale medical studies such as the UK Biobank examine thousands of volunteer participants with medical imaging techniques. Combined with the vast amount of collected metadata, anatomical information from these images has the potential for medical analyses at unprecedented scale. However, their evaluation often requires manual input and long processing times, limiting the amount of reference values for biomarkers and other measurements available for research. Recent approaches with convolutional neural networks for regression can perform these evaluations automatically. On magnetic resonance imaging (MRI) data of more than 40,000 UK Biobank subjects, these systems can estimate human age, body composition and more. This style of analysis is almost entirely data-driven and no manual intervention or guidance with manually segmented ground truth images is required. The networks often closely emulate the reference method that provided their training data and can reach levels of agreement comparable to the expected variability between established medical gold standard techniques. The risk of silent failure can be individually quantified by predictive uncertainty obtained from a mean-variance criterion and ensembling. Saliency analysis furthermore enables an interpretation of the underlying relevant image features and showed that the networks learned to correctly target specific organs, limbs, and regions of interest.
Uncertainty-Aware Body Composition Analysis with Deep Regression Ensembles on UK Biobank MRI
Jan 18, 2021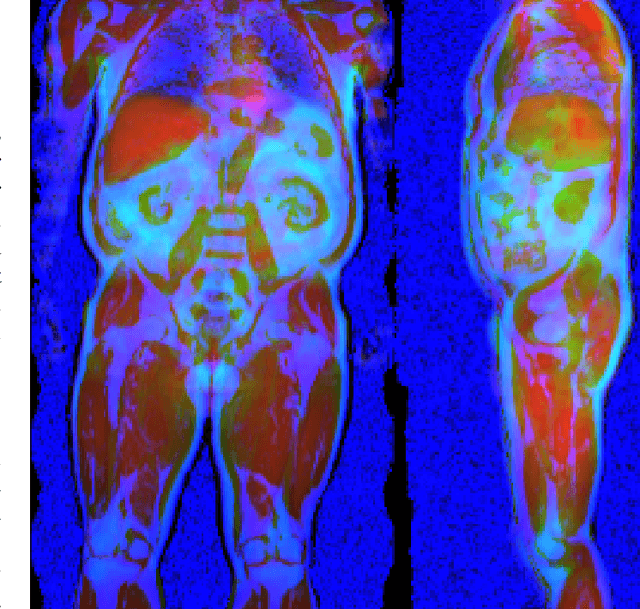
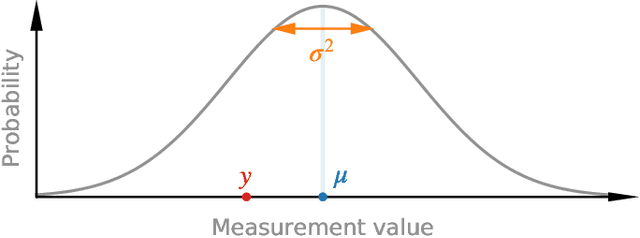
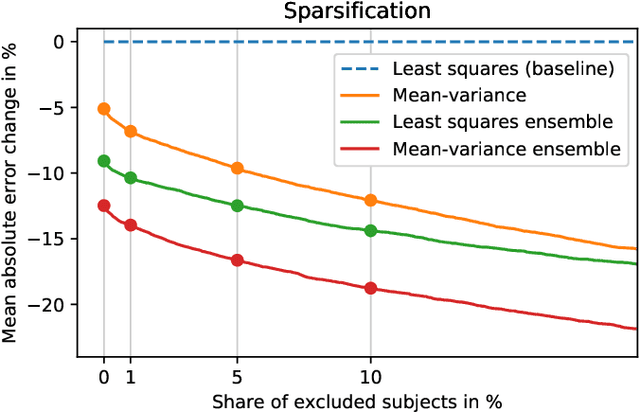
Abstract:Purpose: To enable fast and automated analysis of body composition from UK Biobank MRI with accurate estimates of individual measurement errors. Methods: In an ongoing large-scale imaging study the UK Biobank has acquired MRI of over 40,000 men and women aged 44-82. Phenotypes derived from these images, such as body composition, can reveal new links between genetics, cardiovascular disease, and metabolic conditions. In this retrospective study, neural networks were trained to provide six measurements of body composition from UK Biobank neck-to-knee body MRI. A ResNet50 architecture can automatically predict these values by image-based regression, but may also produce erroneous outliers. Predictive uncertainty, which could identify these failure cases, was therefore modeled with a mean-variance loss and ensembling. Its estimates of individual prediction errors were evaluated in cross-validation on over 8,000 subjects, tested on another 1,000 cases, and finally applied for inference. Results: Relative measurement errors below 5\% were achieved on all but one target, for intra-class correlation coefficients (ICC) above 0.97 both in validation and testing. Both mean-variance loss and ensembling yielded improvements and provided uncertainty estimates that highlighted some of the worst outlier predictions. Combined, they reached the highest quality, but also exhibited a consistent bias towards high uncertainty in heavyweight subjects. Conclusion: Mean-variance regression and ensembling provided complementary benefits for automated body composition measurements from UK Biobank MRI, reaching high speed and accuracy. These values were inferred for the entire cohort, with uncertainty estimates that can approximate the measurement errors and identify some of the worst outliers automatically.
Large-scale inference of liver fat with neural networks on UK Biobank body MRI
Jun 30, 2020
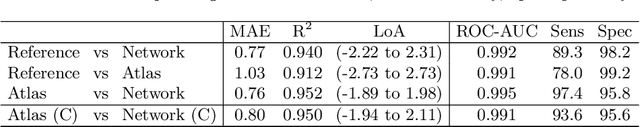
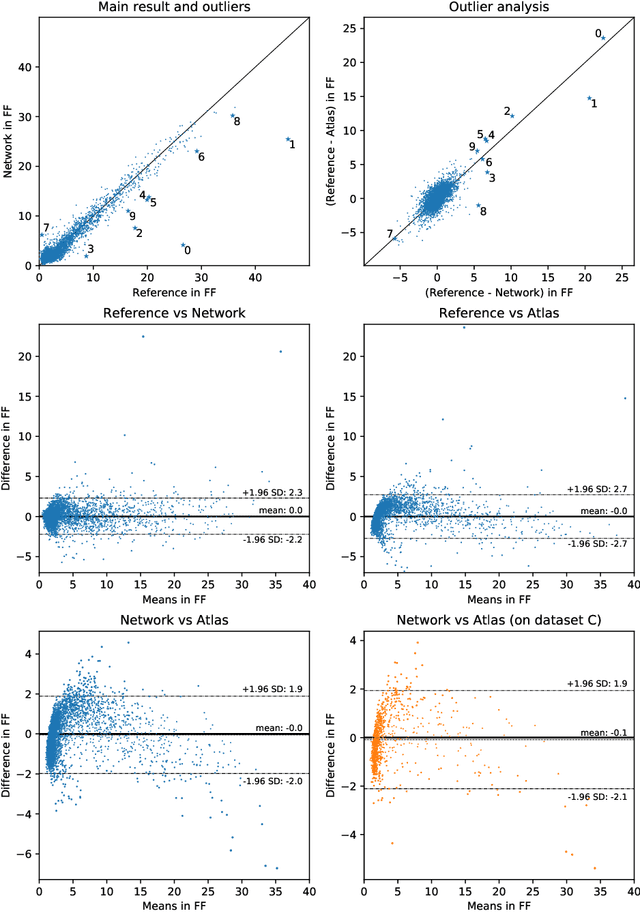
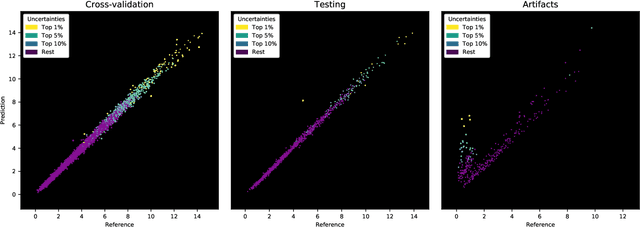
Abstract:The UK Biobank Imaging Study has acquired medical scans of more than 40,000 volunteer participants. The resulting wealth of anatomical information has been made available for research, together with extensive metadata including measurements of liver fat. These values play an important role in metabolic disease, but are only available for a minority of imaged subjects as their collection requires the careful work of image analysts on dedicated liver MRI. Another UK Biobank protocol is neck-to-knee body MRI for analysis of body composition. The resulting volumes can also quantify fat fractions, even though they were reconstructed with a two- instead of a three-point Dixon technique. In this work, a novel framework for automated inference of liver fat from UK Biobank neck-to-knee body MRI is proposed. A ResNet50 was trained for regression on two-dimensional slices from these scans and the reference values as target, without any need for ground truth segmentations. Once trained, it performs fast, objective, and fully automated predictions that require no manual intervention. On the given data, it closely emulates the reference method, reaching a level of agreement comparable to different gold standard techniques. The network learned to rectify non-linearities in the fat fraction values and identified several outliers in the reference. It outperformed a multi-atlas segmentation baseline and inferred new estimates for all imaged subjects lacking reference values, expanding the total number of liver fat measurements by factor six.
Kidney segmentation in neck-to-knee body MRI of 40,000 UK Biobank participants
Jun 12, 2020
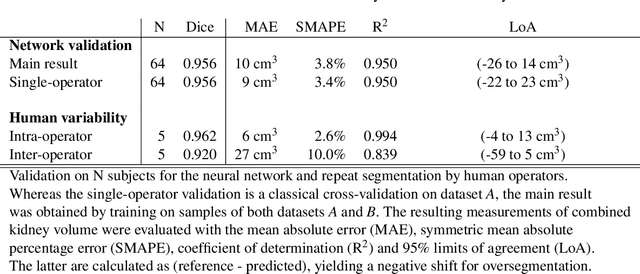
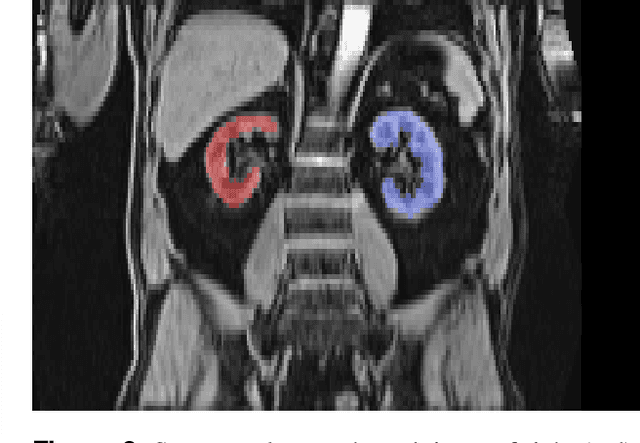
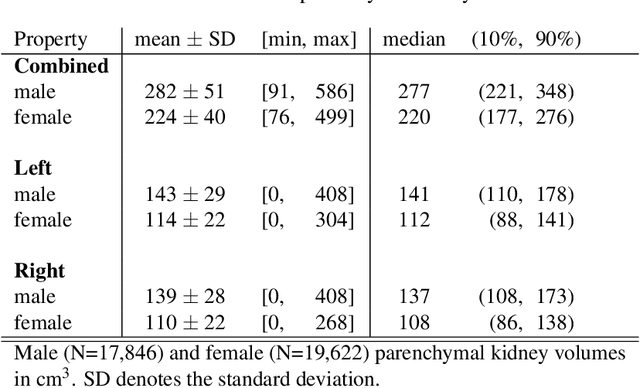
Abstract:The UK Biobank is collecting extensive data on health-related characteristics of over half a million volunteers. The biological samples of blood and urine can provide valuable insight on kidney function, with important links to cardiovascular and metabolic health. Further information on kidney anatomy could be obtained by medical imaging. In contrast to the brain, heart, liver, and pancreas, no dedicated Magnetic Resonance Imaging (MRI) is planned for the kidneys. An image-based assessment is nonetheless feasible in the neck-to-knee body MRI intended for abdominal body composition analysis, which also covers the kidneys. In this work, a pipeline for automated segmentation of parenchymal kidney volume in UK Biobank neck-to-knee body MRI is proposed. The underlying neural network reaches a relative error of 3.8%, with Dice score 0.956 in validation on 64 subjects, close to the 2.6% and Dice score 0.962 for repeated segmentation by one human operator. The released MRI of about 40,000 subjects can be processed within two days, yielding volume measurements of left and right kidney. Algorithmic quality ratings enabled the exclusion of outliers and potential failure cases. The resulting measurements can be studied and shared for large-scale investigation of associations and longitudinal changes in parenchymal kidney volume.
Large-scale biometry with interpretable neural network regression on UK Biobank body MRI
Mar 10, 2020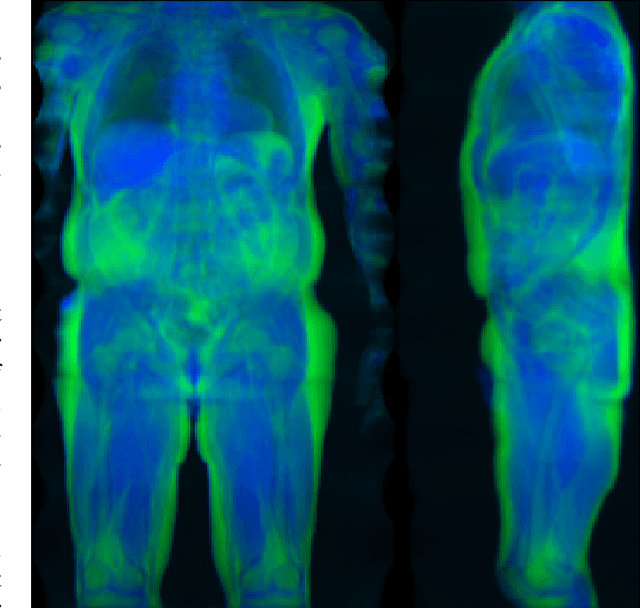
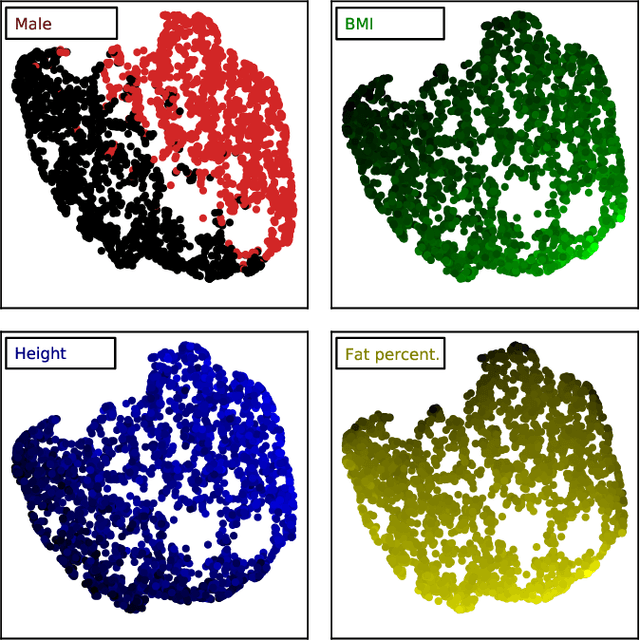
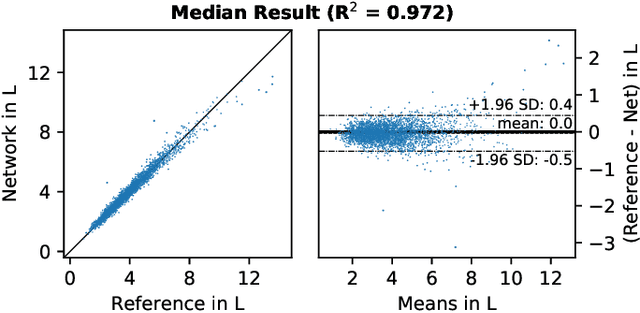
Abstract:Objective: Automated analysis of MRI with deep regression has the potential to provide medical research with a wide range of biological metrics, inferred at high speed and accuracy. Methods: The UK Biobank study has successfully imaged more than 32,000 volunteer participants with neck-to-knee body MRI. Each scan is linked to extensive metadata, providing a comprehensive survey of imaged anatomy and related health states. Despite its potential for research, this vast amount of data presents a challenge to established methods of evaluation, which often rely on manual input. In this work, neural networks were trained for regression to infer various biological metrics from the neck-to-knee body MRI automatically, with a ResNet50 in 7-fold cross-validation. No manual intervention or ground truth segmentations are required for training. The examined fields span 64 variables derived from anthropometric measurements, dual-energy X-ray absorptiometry (DXA), atlas-based segmentations, and dedicated liver scans. Results: The standardized framework achieved a close fit to the target values (median R^2 > 0.97). Interpretation of aggregated saliency maps indicates that the network correctly targets specific body regions and limbs, and learned to emulate different modalities. On several body composition metrics, the quality of the predictions is within the range of variability observed between established gold standard techniques. Conclusion and Significance: The deep regression framework robustly inferred a wide range of medically relevant metrics from the image data. In practice, this technique could provide accurate, image-based measurements for medical research months or years before the more established reference methods have been fully applied.
Separation of water and fat signal in whole-body gradient echo scans using convolutional neural networks
Dec 12, 2018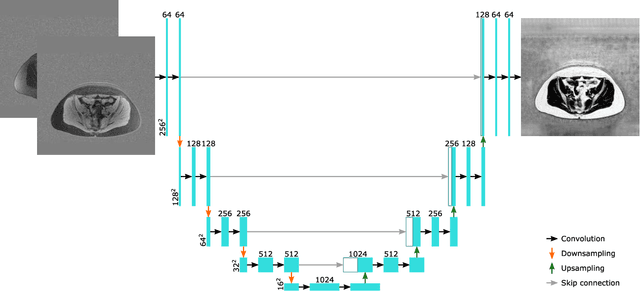

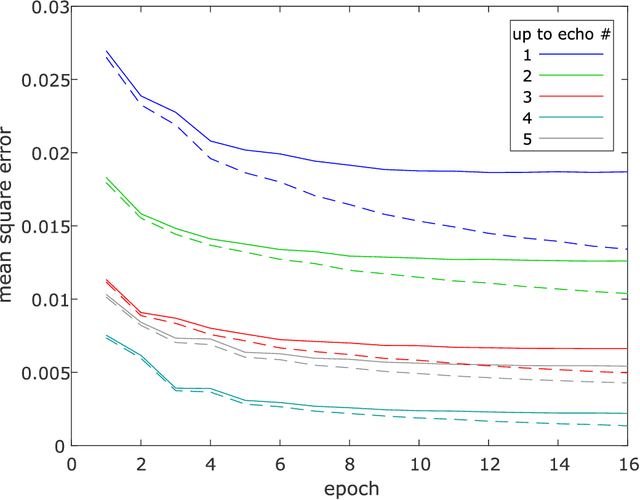

Abstract:Purpose: To perform and evaluate water and fat signal separation of whole-body gradient echo scans using convolutional neural networks. Methods: Whole-body gradient echo scans of 240 subjects, each consisting of five bipolar echoes, were used. Reference fat fraction maps were created using a conventional method. Convolutional neural networks, more specifically 2D U-nets, were trained using 5-fold cross-validation with one or several echoes as input, using the squared difference between the output and the reference fat fraction maps as the loss function. The outputs of the networks were assessed by the loss function, measured liver fat fractions, and visually. Training was performed using a GPU. Inference was performed both using the GPU as well as a CPU. Results: The final loss of the validation data decreased when using more echoes as input, and the loss curves indicated convergence. The liver fat fractions could be estimated using only one echo, but results were improved by use of more echoes. Visual assessment found the quality of the outputs of the networks to be similar to the reference even when using only one echo, with slight improvements when using more echoes. Training a network took at most 28.6 h. Inference time of a whole-body scan took at most 3.7 s using the GPU and 5.8 min using the CPU. Conclusion: It is possible to perform water and fat signal separation of whole-body gradient echo scans using convolutional neural networks. Separation was possible using only one echo, although using more echoes improved the results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge