Gitaek Kwon
DRAC: Diabetic Retinopathy Analysis Challenge with Ultra-Wide Optical Coherence Tomography Angiography Images
Apr 05, 2023



Abstract:Computer-assisted automatic analysis of diabetic retinopathy (DR) is of great importance in reducing the risks of vision loss and even blindness. Ultra-wide optical coherence tomography angiography (UW-OCTA) is a non-invasive and safe imaging modality in DR diagnosis system, but there is a lack of publicly available benchmarks for model development and evaluation. To promote further research and scientific benchmarking for diabetic retinopathy analysis using UW-OCTA images, we organized a challenge named "DRAC - Diabetic Retinopathy Analysis Challenge" in conjunction with the 25th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2022). The challenge consists of three tasks: segmentation of DR lesions, image quality assessment and DR grading. The scientific community responded positively to the challenge, with 11, 12, and 13 teams from geographically diverse institutes submitting different solutions in these three tasks, respectively. This paper presents a summary and analysis of the top-performing solutions and results for each task of the challenge. The obtained results from top algorithms indicate the importance of data augmentation, model architecture and ensemble of networks in improving the performance of deep learning models. These findings have the potential to enable new developments in diabetic retinopathy analysis. The challenge remains open for post-challenge registrations and submissions for benchmarking future methodology developments.
Bag of Tricks for Developing Diabetic Retinopathy Analysis Framework to Overcome Data Scarcity
Oct 18, 2022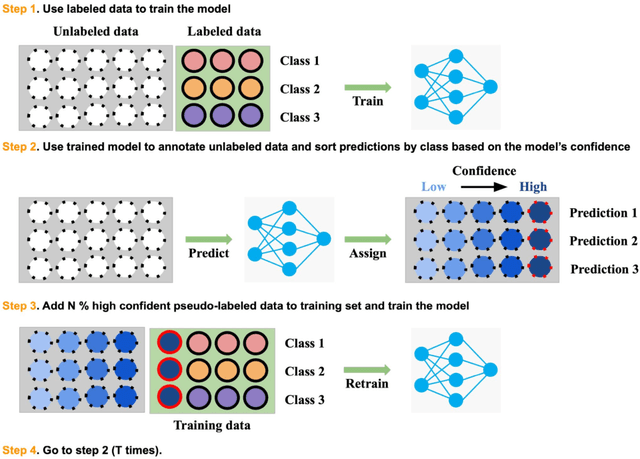
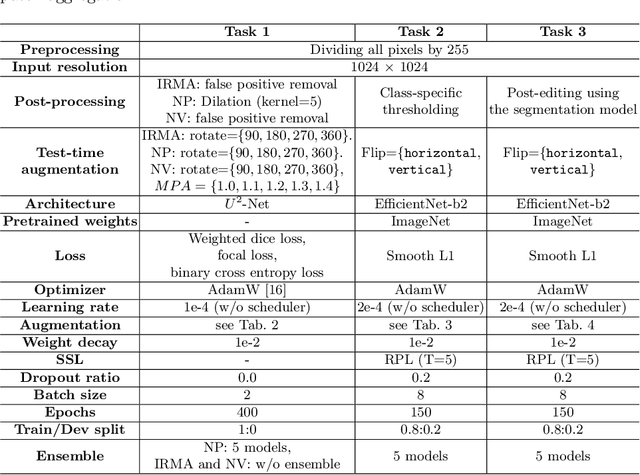
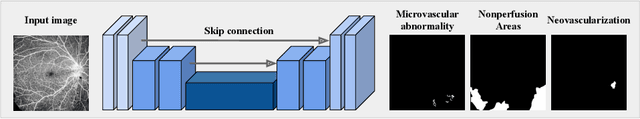
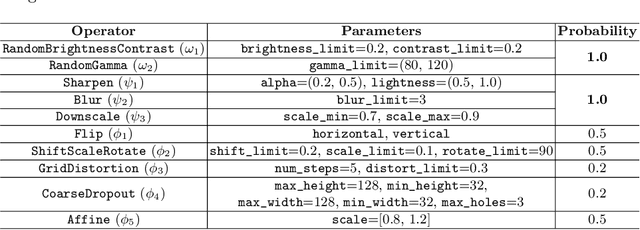
Abstract:Recently, diabetic retinopathy (DR) screening utilizing ultra-wide optical coherence tomography angiography (UW-OCTA) has been used in clinical practices to detect signs of early DR. However, developing a deep learning-based DR analysis system using UW-OCTA images is not trivial due to the difficulty of data collection and the absence of public datasets. By realistic constraints, a model trained on small datasets may obtain sub-par performance. Therefore, to help ophthalmologists be less confused about models' incorrect decisions, the models should be robust even in data scarcity settings. To address the above practical challenging, we present a comprehensive empirical study for DR analysis tasks, including lesion segmentation, image quality assessment, and DR grading. For each task, we introduce a robust training scheme by leveraging ensemble learning, data augmentation, and semi-supervised learning. Furthermore, we propose reliable pseudo labeling that excludes uncertain pseudo-labels based on the model's confidence scores to reduce the negative effect of noisy pseudo-labels. By exploiting the proposed approaches, we achieved 1st place in the Diabetic Retinopathy Analysis Challenge.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge