Giorgio Dolci
Department of Computer Science, University of Verona, Verona, Italy, Department of Engineering for Innovation Medicine, University of Verona, Verona, Italy, Tri-Institutional Center for Translational Research in Neuroimaging and Data Science
Guidelines For The Choice Of The Baseline in XAI Attribution Methods
Mar 25, 2025Abstract:Given the broad adoption of artificial intelligence, it is essential to provide evidence that AI models are reliable, trustable, and fair. To this end, the emerging field of eXplainable AI develops techniques to probe such requirements, counterbalancing the hype pushing the pervasiveness of this technology. Among the many facets of this issue, this paper focuses on baseline attribution methods, aiming at deriving a feature attribution map at the network input relying on a "neutral" stimulus usually called "baseline". The choice of the baseline is crucial as it determines the explanation of the network behavior. In this framework, this paper has the twofold goal of shedding light on the implications of the choice of the baseline and providing a simple yet effective method for identifying the best baseline for the task. To achieve this, we propose a decision boundary sampling method, since the baseline, by definition, lies on the decision boundary, which naturally becomes the search domain. Experiments are performed on synthetic examples and validated relying on state-of-the-art methods. Despite being limited to the experimental scope, this contribution is relevant as it offers clear guidelines and a simple proxy for baseline selection, reducing ambiguity and enhancing deep models' reliability and trust.
Multimodal MRI-based Detection of Amyloid Status in Alzheimer's Disease Continuum
Jun 19, 2024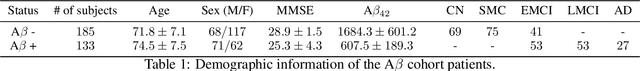
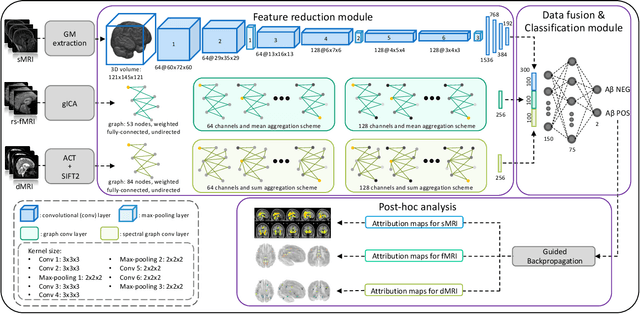

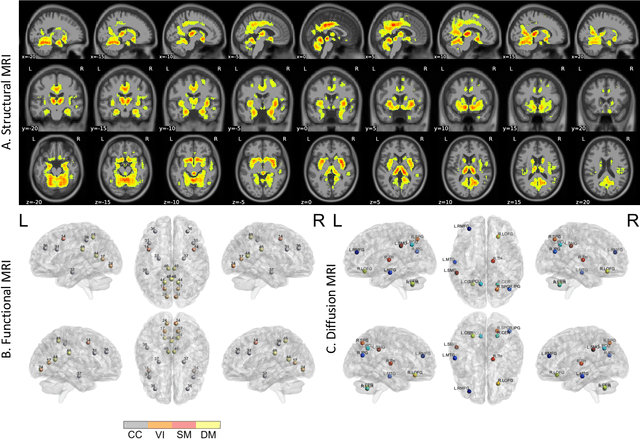
Abstract:Amyloid-$\beta$ (A$\beta$) plaques in conjunction with hyperphosphorylated tau proteins in the form of neurofibrillary tangles are the two neuropathological hallmarks of Alzheimer's disease (AD). In particular, the accumulation of A$\beta$ plaques, as evinced by the A/T/N (amyloid/tau/neurodegeneration) framework, marks the initial stage. Thus, the identification of individuals with A$\beta$ positivity could enable early diagnosis and potentially lead to more effective interventions. Deep learning methods relying mainly on amyloid PET images have been employed to this end. However, PET imaging has some disadvantages, including the need of radiotracers and expensive acquisitions. Hence, in this work, we propose a novel multimodal approach that integrates information from structural, functional, and diffusion MRI data to discriminate A$\beta$ status in the AD continuum. Our method achieved an accuracy of $0.762\pm0.04$. Furthermore, a \textit{post-hoc} explainability analysis (guided backpropagation) was performed to retrieve the brain regions that most influenced the model predictions. This analysis identified some key regions that were common across modalities, some of which were well-established AD-discriminative biomarkers and related to A$\beta$ deposition, such as the hippocampus, thalamus, precuneus, and cingulate gyrus. Hence, our study demonstrates the potential viability of MRI-based characterization of A$\beta$ status, paving the way for further research in this domain.
An interpretable generative multimodal neuroimaging-genomics framework for decoding Alzheimer's disease
Jun 19, 2024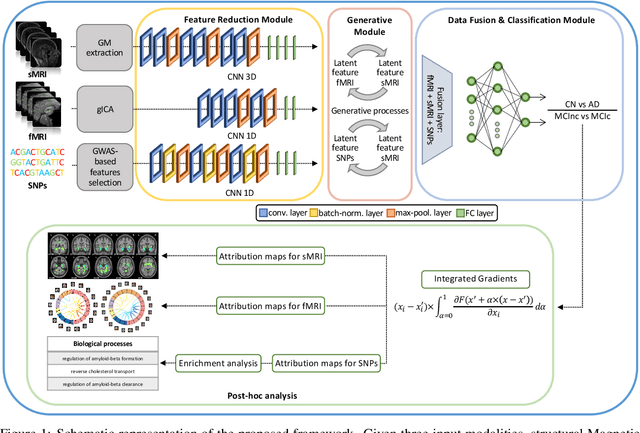

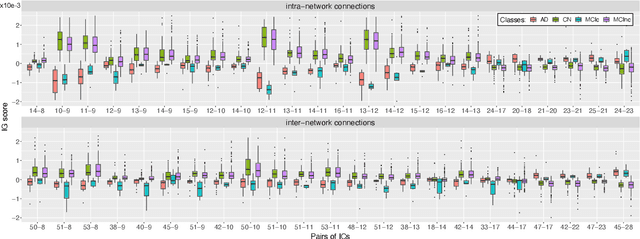

Abstract:Alzheimer's disease (AD) is the most prevalent form of dementia with a progressive decline in cognitive abilities. The AD continuum encompasses a prodormal stage known as Mild Cognitive Impairment (MCI), where patients may either progress to AD or remain stable. In this study, we leveraged structural and functional MRI to investigate the disease-induced grey matter and functional network connectivity changes. Moreover, considering AD's strong genetic component, we introduce SNPs as a third channel. Given such diverse inputs, missing one or more modalities is a typical concern of multimodal methods. We hence propose a novel deep learning-based classification framework where generative module employing Cycle GANs was adopted to impute missing data within the latent space. Additionally, we adopted an Explainable AI method, Integrated Gradients, to extract input features relevance, enhancing our understanding of the learned representations. Two critical tasks were addressed: AD detection and MCI conversion prediction. Experimental results showed that our model was able to reach the SOA in the classification of CN/AD reaching an average test accuracy of $0.926\pm0.02$. For the MCI task, we achieved an average prediction accuracy of $0.711\pm0.01$ using the pre-trained model for CN/AD. The interpretability analysis revealed significant grey matter modulations in cortical and subcortical brain areas well known for their association with AD. Moreover, impairments in sensory-motor and visual resting state network connectivity along the disease continuum, as well as mutations in SNPs defining biological processes linked to amyloid-beta and cholesterol formation clearance and regulation, were identified as contributors to the achieved performance. Overall, our integrative deep learning approach shows promise for AD detection and MCI prediction, while shading light on important biological insights.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge