Md Abdur Rahaman
Tri-Institutional Center for Translational Research in Neuroimaging and Data Science
An interpretable generative multimodal neuroimaging-genomics framework for decoding Alzheimer's disease
Jun 19, 2024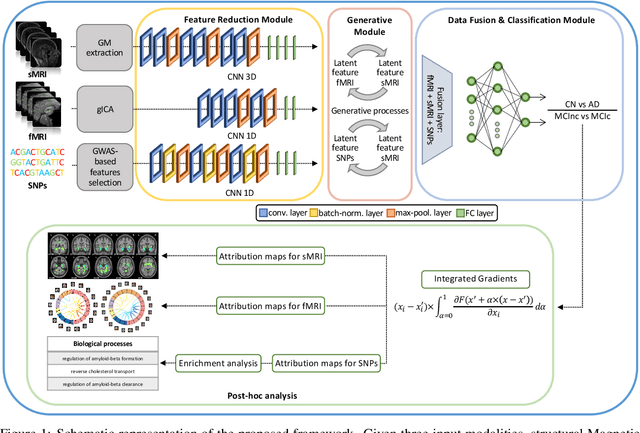

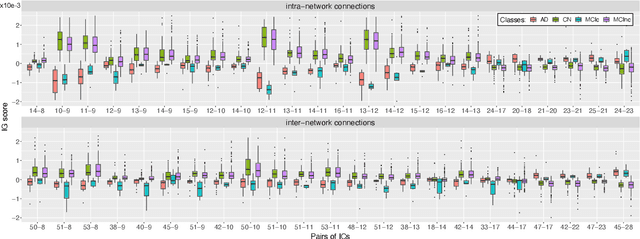

Abstract:Alzheimer's disease (AD) is the most prevalent form of dementia with a progressive decline in cognitive abilities. The AD continuum encompasses a prodormal stage known as Mild Cognitive Impairment (MCI), where patients may either progress to AD or remain stable. In this study, we leveraged structural and functional MRI to investigate the disease-induced grey matter and functional network connectivity changes. Moreover, considering AD's strong genetic component, we introduce SNPs as a third channel. Given such diverse inputs, missing one or more modalities is a typical concern of multimodal methods. We hence propose a novel deep learning-based classification framework where generative module employing Cycle GANs was adopted to impute missing data within the latent space. Additionally, we adopted an Explainable AI method, Integrated Gradients, to extract input features relevance, enhancing our understanding of the learned representations. Two critical tasks were addressed: AD detection and MCI conversion prediction. Experimental results showed that our model was able to reach the SOA in the classification of CN/AD reaching an average test accuracy of $0.926\pm0.02$. For the MCI task, we achieved an average prediction accuracy of $0.711\pm0.01$ using the pre-trained model for CN/AD. The interpretability analysis revealed significant grey matter modulations in cortical and subcortical brain areas well known for their association with AD. Moreover, impairments in sensory-motor and visual resting state network connectivity along the disease continuum, as well as mutations in SNPs defining biological processes linked to amyloid-beta and cholesterol formation clearance and regulation, were identified as contributors to the achieved performance. Overall, our integrative deep learning approach shows promise for AD detection and MCI prediction, while shading light on important biological insights.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge