Charles A. Ellis
Tri-Institutional Center for Translational Research in Neuroimaging and Data Science
Multimodal MRI-based Detection of Amyloid Status in Alzheimer's Disease Continuum
Jun 19, 2024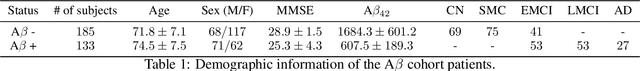
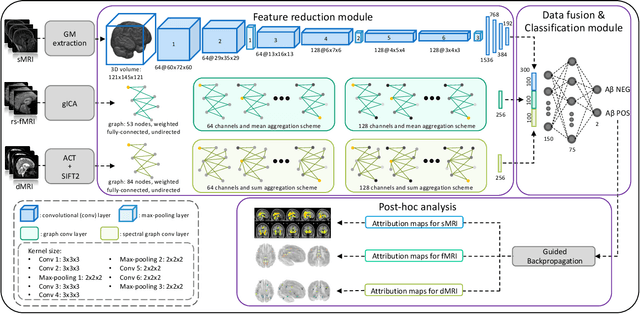

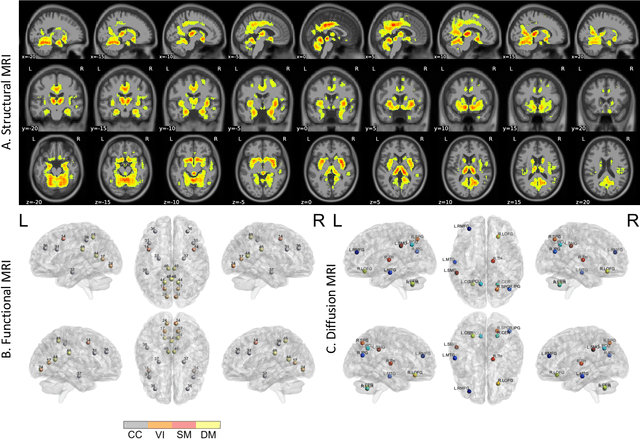
Abstract:Amyloid-$\beta$ (A$\beta$) plaques in conjunction with hyperphosphorylated tau proteins in the form of neurofibrillary tangles are the two neuropathological hallmarks of Alzheimer's disease (AD). In particular, the accumulation of A$\beta$ plaques, as evinced by the A/T/N (amyloid/tau/neurodegeneration) framework, marks the initial stage. Thus, the identification of individuals with A$\beta$ positivity could enable early diagnosis and potentially lead to more effective interventions. Deep learning methods relying mainly on amyloid PET images have been employed to this end. However, PET imaging has some disadvantages, including the need of radiotracers and expensive acquisitions. Hence, in this work, we propose a novel multimodal approach that integrates information from structural, functional, and diffusion MRI data to discriminate A$\beta$ status in the AD continuum. Our method achieved an accuracy of $0.762\pm0.04$. Furthermore, a \textit{post-hoc} explainability analysis (guided backpropagation) was performed to retrieve the brain regions that most influenced the model predictions. This analysis identified some key regions that were common across modalities, some of which were well-established AD-discriminative biomarkers and related to A$\beta$ deposition, such as the hippocampus, thalamus, precuneus, and cingulate gyrus. Hence, our study demonstrates the potential viability of MRI-based characterization of A$\beta$ status, paving the way for further research in this domain.
Improving age prediction: Utilizing LSTM-based dynamic forecasting for data augmentation in multivariate time series analysis
Dec 11, 2023

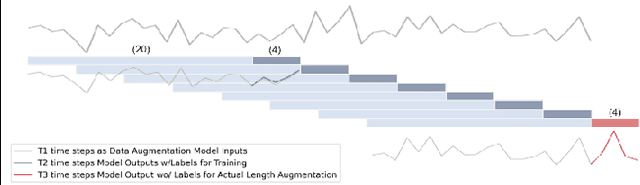

Abstract:The high dimensionality and complexity of neuroimaging data necessitate large datasets to develop robust and high-performing deep learning models. However, the neuroimaging field is notably hampered by the scarcity of such datasets. In this work, we proposed a data augmentation and validation framework that utilizes dynamic forecasting with Long Short-Term Memory (LSTM) networks to enrich datasets. We extended multivariate time series data by predicting the time courses of independent component networks (ICNs) in both one-step and recursive configurations. The effectiveness of these augmented datasets was then compared with the original data using various deep learning models designed for chronological age prediction tasks. The results suggest that our approach improves model performance, providing a robust solution to overcome the challenges presented by the limited size of neuroimaging datasets.
Algorithm-Agnostic Explainability for Unsupervised Clustering
May 17, 2021
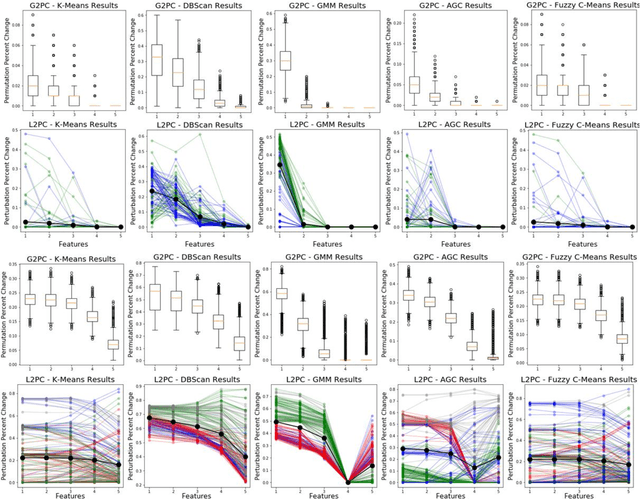
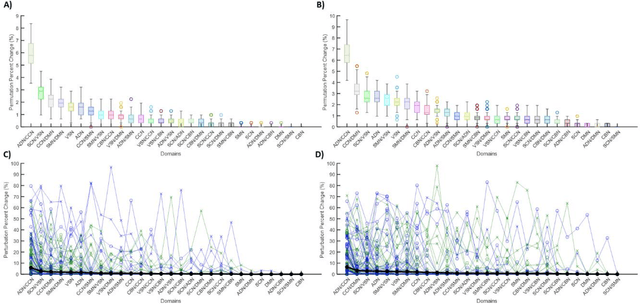
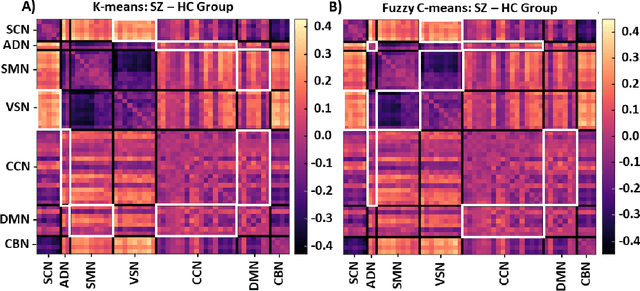
Abstract:Supervised machine learning explainability has greatly expanded in recent years. However, the field of unsupervised clustering explainability has lagged behind. Here, we, to the best of our knowledge, demonstrate for the first time how model-agnostic methods for supervised machine learning explainability can be adapted to provide algorithm-agnostic unsupervised clustering explainability. We present two novel algorithm-agnostic explainability methods, global permutation percent change (G2PC) feature importance and local perturbation percent change (L2PC) feature importance, that can provide insight into many clustering methods on a global level by identifying the relative importance of features to a clustering algorithm and on a local level by identifying the relative importance of features to the clustering of individual samples. We demonstrate the utility of the methods for explaining five popular clustering algorithms on low-dimensional, ground-truth synthetic datasets and on high-dimensional functional network connectivity (FNC) data extracted from a resting state functional magnetic resonance imaging (rs-fMRI) dataset of 151 subjects with schizophrenia (SZ) and 160 healthy controls (HC). Our proposed explainability methods robustly identify the relative importance of features across multiple clustering methods and could facilitate new insights into many applications. We hope that this study will greatly accelerate the development of the field of clustering explainability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge