Federico Pedersoli
Predicting Osteoarthritis Progression in Radiographs via Unsupervised Representation Learning
Nov 22, 2021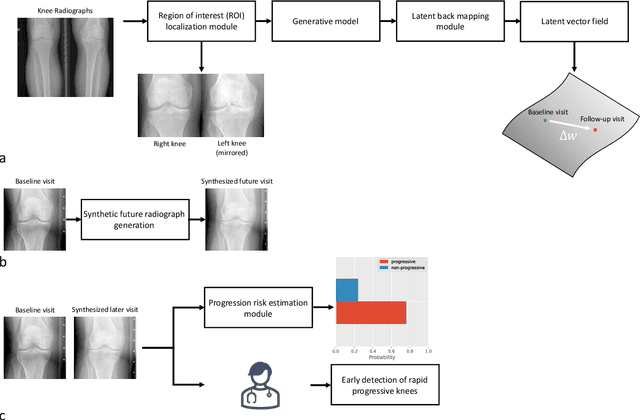
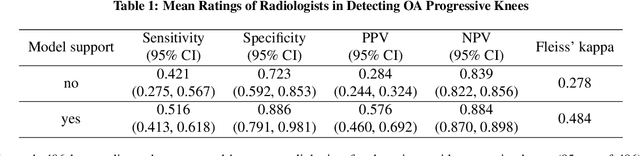
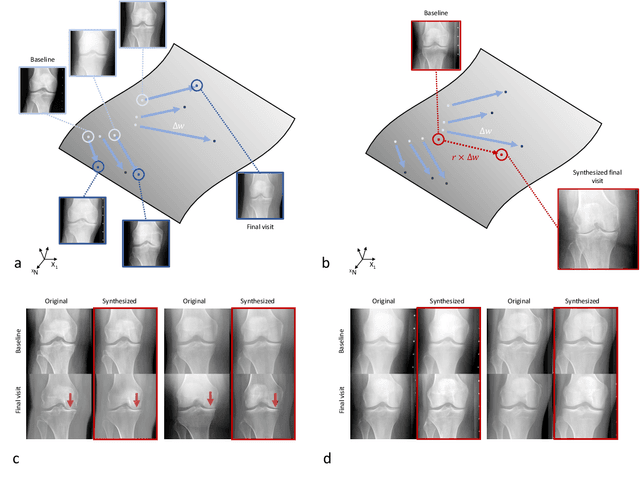
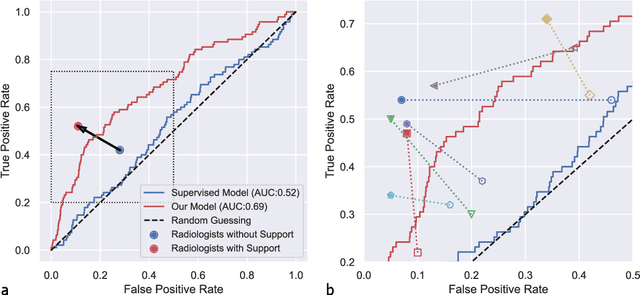
Abstract:Osteoarthritis (OA) is the most common joint disorder affecting substantial proportions of the global population, primarily the elderly. Despite its individual and socioeconomic burden, the onset and progression of OA can still not be reliably predicted. Aiming to fill this diagnostic gap, we introduce an unsupervised learning scheme based on generative models to predict the future development of OA based on knee joint radiographs. Using longitudinal data from osteoarthritis studies, we explore the latent temporal trajectory to predict a patient's future radiographs up to the eight-year follow-up visit. Our model predicts the risk of progression towards OA and surpasses its supervised counterpart whose input was provided by seven experienced radiologists. With the support of the model, sensitivity, specificity, positive predictive value, and negative predictive value increased significantly from 42.1% to 51.6%, from 72.3% to 88.6%, from 28.4% to 57.6%, and from 83.9% to 88.4%, respectively, while without such support, radiologists performed only slightly better than random guessing. Our predictive model improves predictions on OA onset and progression, despite requiring no human annotation in the training phase.
Advancing diagnostic performance and clinical usability of neural networks via adversarial training and dual batch normalization
Nov 25, 2020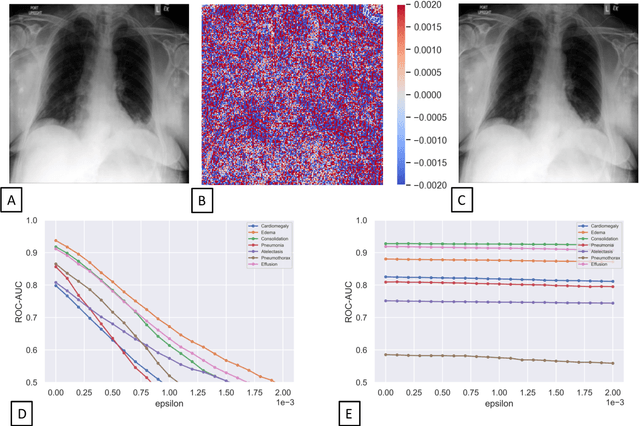
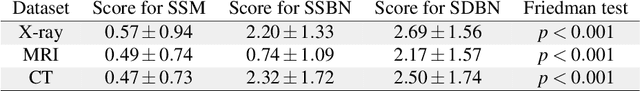
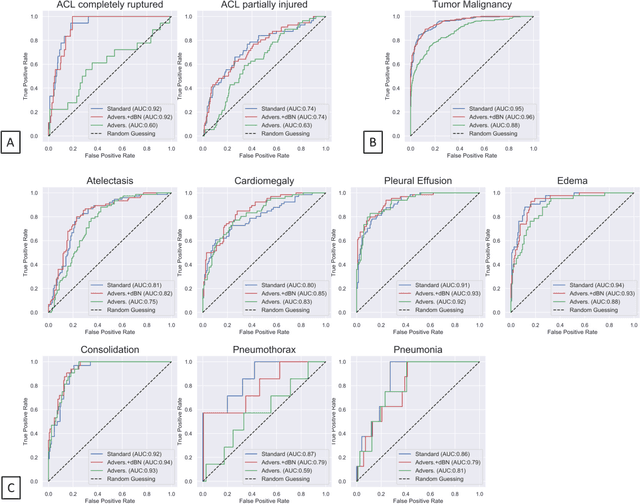

Abstract:Unmasking the decision-making process of machine learning models is essential for implementing diagnostic support systems in clinical practice. Here, we demonstrate that adversarially trained models can significantly enhance the usability of pathology detection as compared to their standard counterparts. We let six experienced radiologists rate the interpretability of saliency maps in datasets of X-rays, computed tomography, and magnetic resonance imaging scans. Significant improvements were found for our adversarial models, which could be further improved by the application of dual batch normalization. Contrary to previous research on adversarially trained models, we found that the accuracy of such models was equal to standard models when sufficiently large datasets and dual batch norm training were used. To ensure transferability, we additionally validated our results on an external test set of 22,433 X-rays. These findings elucidate that different paths for adversarial and real images are needed during training to achieve state of the art results with superior clinical interpretability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge