Fanwei Kong
Full-field surrogate modeling of cardiac function encoding geometric variability
Apr 29, 2025Abstract:Combining physics-based modeling with data-driven methods is critical to enabling the translation of computational methods to clinical use in cardiology. The use of rigorous differential equations combined with machine learning tools allows for model personalization with uncertainty quantification in time frames compatible with clinical practice. However, accurate and efficient surrogate models of cardiac function, built from physics-based numerical simulation, are still mostly geometry-specific and require retraining for different patients and pathological conditions. We propose a novel computational pipeline to embed cardiac anatomies into full-field surrogate models. We generate a dataset of electrophysiology simulations using a complex multi-scale mathematical model coupling partial and ordinary differential equations. We adopt Branched Latent Neural Maps (BLNMs) as an effective scientific machine learning method to encode activation maps extracted from physics-based numerical simulations into a neural network. Leveraging large deformation diffeomorphic metric mappings, we build a biventricular anatomical atlas and parametrize the anatomical variability of a small and challenging cohort of 13 pediatric patients affected by Tetralogy of Fallot. We propose a novel statistical shape modeling based z-score sampling approach to generate a new synthetic cohort of 52 biventricular geometries that are compatible with the original geometrical variability. This synthetic cohort acts as the training set for BLNMs. Our surrogate model demonstrates robustness and great generalization across the complex original patient cohort, achieving an average adimensional mean squared error of 0.0034. The Python implementation of our BLNM model is publicly available under MIT License at https://github.com/StanfordCBCL/BLNM.
SDF4CHD: Generative Modeling of Cardiac Anatomies with Congenital Heart Defects
Nov 08, 2023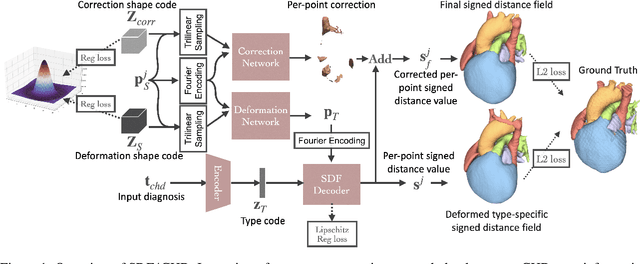
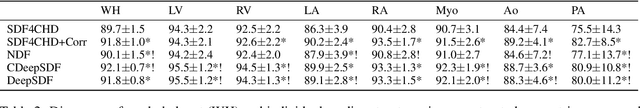
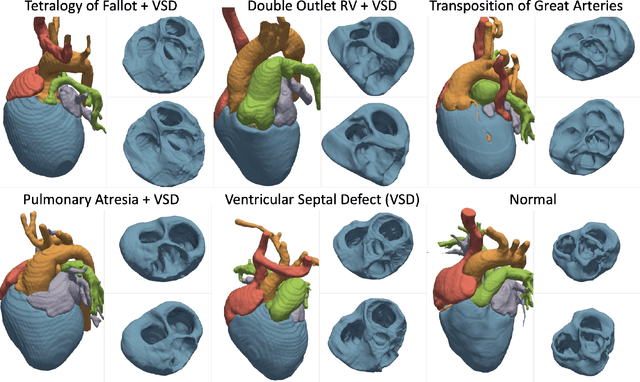
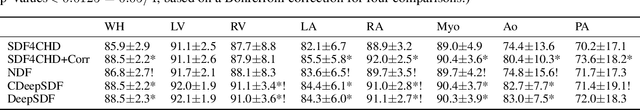
Abstract:Congenital heart disease (CHD) encompasses a spectrum of cardiovascular structural abnormalities, often requiring customized treatment plans for individual patients. Computational modeling and analysis of these unique cardiac anatomies can improve diagnosis and treatment planning and may ultimately lead to improved outcomes. Deep learning (DL) methods have demonstrated the potential to enable efficient treatment planning by automating cardiac segmentation and mesh construction for patients with normal cardiac anatomies. However, CHDs are often rare, making it challenging to acquire sufficiently large patient cohorts for training such DL models. Generative modeling of cardiac anatomies has the potential to fill this gap via the generation of virtual cohorts; however, prior approaches were largely designed for normal anatomies and cannot readily capture the significant topological variations seen in CHD patients. Therefore, we propose a type- and shape-disentangled generative approach suitable to capture the wide spectrum of cardiac anatomies observed in different CHD types and synthesize differently shaped cardiac anatomies that preserve the unique topology for specific CHD types. Our DL approach represents generic whole heart anatomies with CHD type-specific abnormalities implicitly using signed distance fields (SDF) based on CHD type diagnosis, which conveniently captures divergent anatomical variations across different types and represents meaningful intermediate CHD states. To capture the shape-specific variations, we then learn invertible deformations to morph the learned CHD type-specific anatomies and reconstruct patient-specific shapes. Our approach has the potential to augment the image-segmentation pairs for rarer CHD types for cardiac segmentation and generate cohorts of CHD cardiac meshes for computational simulation.
LinFlo-Net: A two-stage deep learning method to generate simulation ready meshes of the heart
Oct 30, 2023Abstract:We present a deep learning model to automatically generate computer models of the human heart from patient imaging data with an emphasis on its capability to generate thin-walled cardiac structures. Our method works by deforming a template mesh to fit the cardiac structures to the given image. Compared with prior deep learning methods that adopted this approach, our framework is designed to minimize mesh self-penetration, which typically arises when deforming surface meshes separated by small distances. We achieve this by using a two-stage diffeomorphic deformation process along with a novel loss function derived from the kinematics of motion that penalizes surface contact and interpenetration. Our model demonstrates comparable accuracy with state-of-the-art methods while additionally producing meshes free of self-intersections. The resultant meshes are readily usable in physics based simulation, minimizing the need for post-processing and cleanup.
Learning Whole Heart Mesh Generation From Patient Images For Computational Simulations
Mar 20, 2022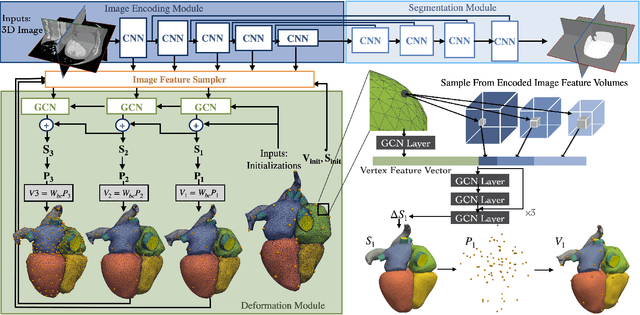
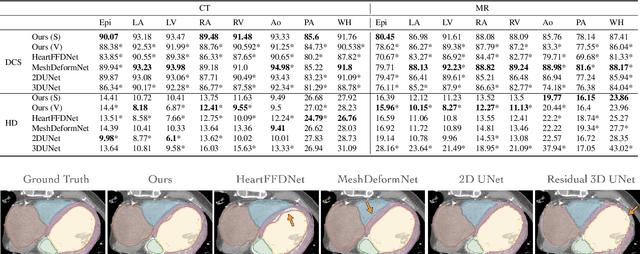
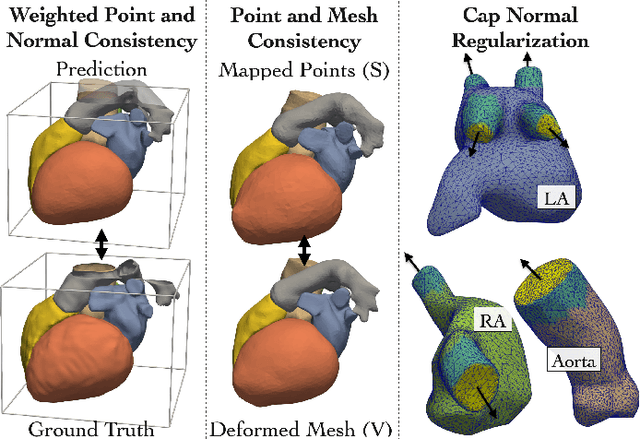
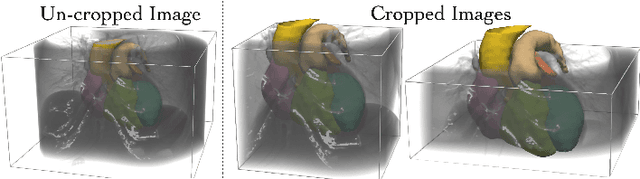
Abstract:Patient-specific cardiac modeling combines geometries of the heart derived from medical images and biophysical simulations to predict various aspects of cardiac function. However, generating simulation-suitable models of the heart from patient image data often requires complicated procedures and significant human effort. We present a fast and automated deep-learning method to construct simulation-suitable models of the heart from medical images. The approach constructs meshes from 3D patient images by learning to deform a small set of deformation handles on a whole heart template. For both 3D CT and MR data, this method achieves promising accuracy for whole heart reconstruction, consistently outperforming prior methods in constructing simulation-suitable meshes of the heart. When evaluated on time-series CT data, this method produced more anatomically and temporally consistent geometries than prior methods, and was able to produce geometries that better satisfy modeling requirements for cardiac flow simulations. Our source code will be available on GitHub.
Whole Heart Mesh Generation For Image-Based Computational Simulations By Learning Free-From Deformations
Jul 22, 2021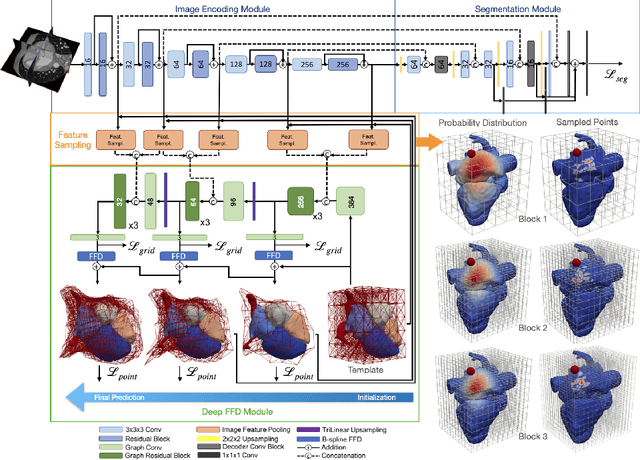
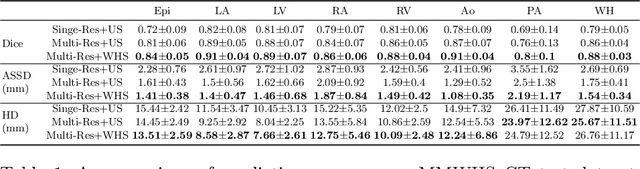
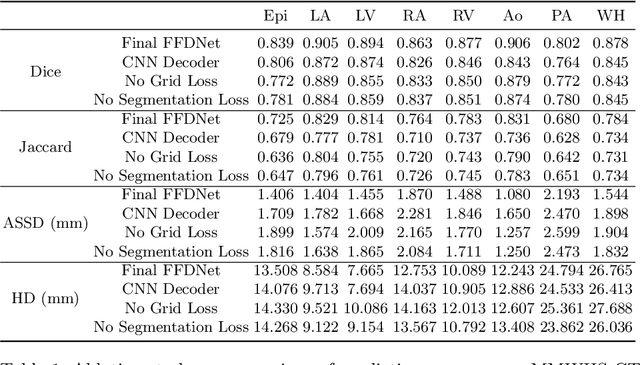
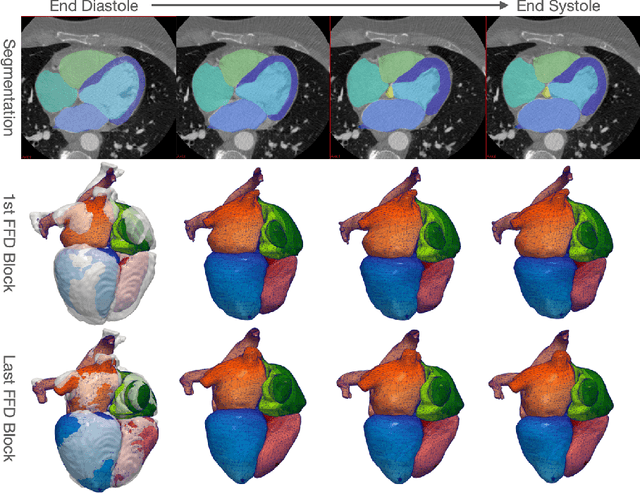
Abstract:Image-based computer simulation of cardiac function can be used to probe the mechanisms of (patho)physiology, and guide diagnosis and personalized treatment of cardiac diseases. This paradigm requires constructing simulation-ready meshes of cardiac structures from medical image data--a process that has traditionally required significant time and human effort, limiting large-cohort analyses and potential clinical translations. We propose a novel deep learning approach to reconstruct simulation-ready whole heart meshes from volumetric image data. Our approach learns to deform a template mesh to the input image data by predicting displacements of multi-resolution control point grids. We discuss the methods of this approach and demonstrate its application to efficiently create simulation-ready whole heart meshes for computational fluid dynamics simulations of the cardiac flow. Our source code is available at https://github.com/fkong7/HeartFFDNet.
A Deep-Learning Approach For Direct Whole-Heart Mesh Reconstruction
Feb 16, 2021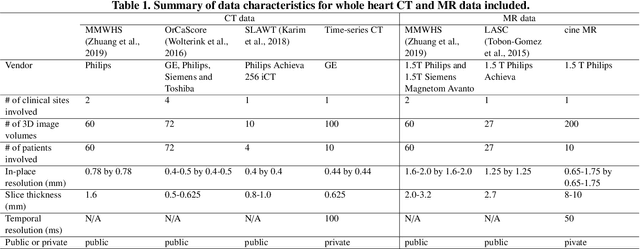
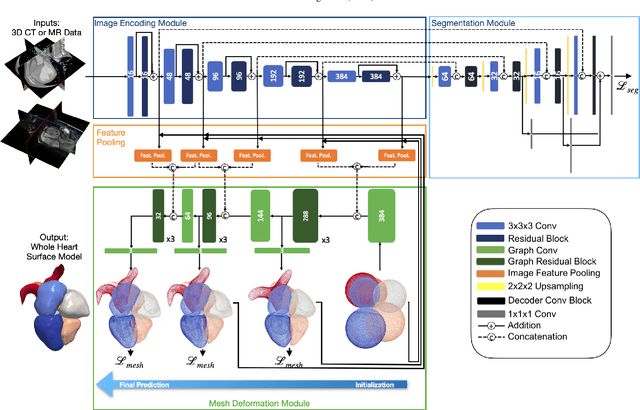
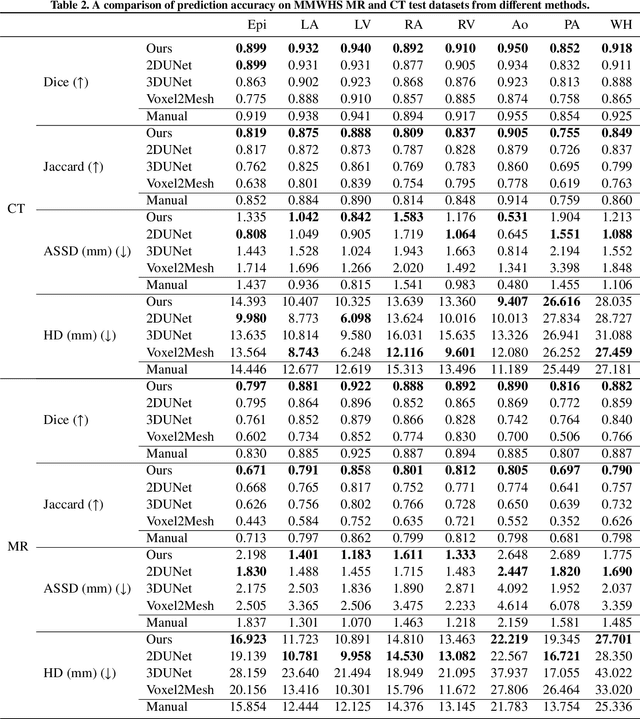
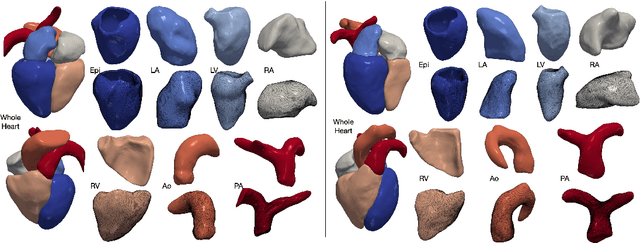
Abstract:Automated construction of surface geometries of cardiac structures from volumetric medical images is important for a number of clinical applications. While deep-learning based approaches have demonstrated promising reconstruction precision, these approaches have mostly focused on voxel-wise segmentation followed by surface reconstruction and post-processing techniques. However, such approaches suffer from a number of limitations including disconnected regions or incorrect surface topology due to erroneous segmentation and stair-case artifacts due to limited segmentation resolution. We propose a novel deep-learning-based approach that directly predicts whole heart surface meshes from volumetric CT and MR image data. Our approach leverages a graph convolutional neural network to predict deformation on mesh vertices from a pre-defined mesh template to reconstruct multiple anatomical structures in a 3D image volume. Our method demonstrated promising performance of generating high-resolution and high-quality whole heart reconstructions and outperformed prior deep-learning based methods on both CT and MR data in terms of precision and surface quality. Furthermore, our method can more efficiently produce temporally-consistent and feature-corresponding surface mesh predictions for heart motion from CT or MR cine sequences, and therefore can potentially be applied for efficiently constructing 4D whole heart dynamics.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge