Emily K. Funk
HemoSet: The First Blood Segmentation Dataset for Automation of Hemostasis Management
Mar 24, 2024



Abstract:Hemorrhaging occurs in surgeries of all types, forcing surgeons to quickly adapt to the visual interference that results from blood rapidly filling the surgical field. Introducing automation into the crucial surgical task of hemostasis management would offload mental and physical tasks from the surgeon and surgical assistants while simultaneously increasing the efficiency and safety of the operation. The first step in automation of hemostasis management is detection of blood in the surgical field. To propel the development of blood detection algorithms in surgeries, we present HemoSet, the first blood segmentation dataset based on bleeding during a live animal robotic surgery. Our dataset features vessel hemorrhage scenarios where turbulent flow leads to abnormal pooling geometries in surgical fields. These pools are formed in conditions endemic to surgical procedures -- uneven heterogeneous tissue, under glossy lighting conditions and rapid tool movement. We benchmark several state-of-the-art segmentation models and provide insight into the difficulties specific to blood detection. We intend for HemoSet to spur development of autonomous blood suction tools by providing a platform for training and refining blood segmentation models, addressing the precision needed for such robotics.
From Bench to Bedside: The First Live Robotic Surgery on the dVRK to Enable Remote Telesurgery with Motion Scaling
Sep 24, 2021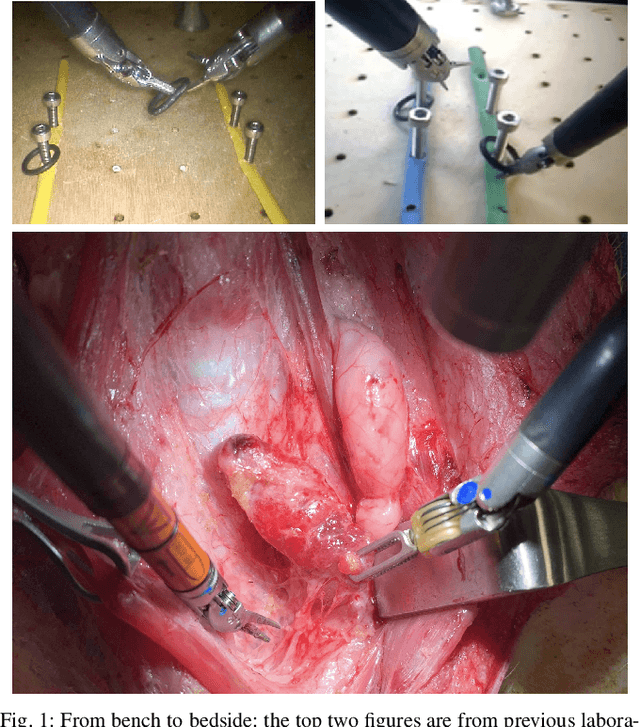
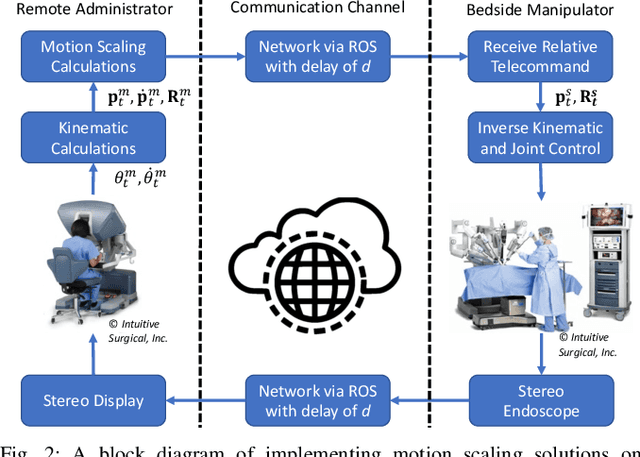
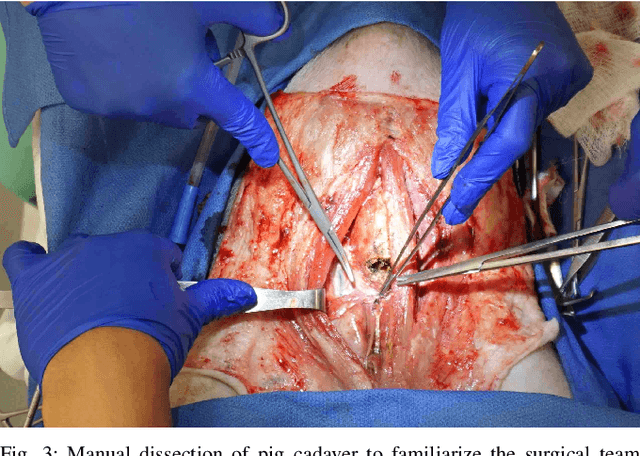
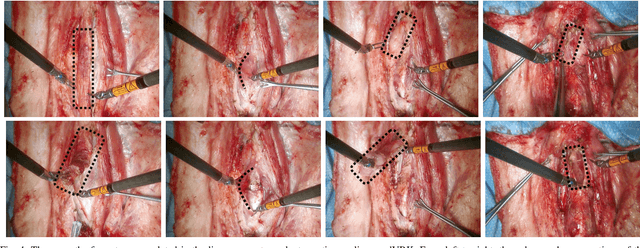
Abstract:Innovations from surgical robotic research rarely translates to live surgery due to the significant difference between the lab and a live environment. Live environments require considerations that are often overlooked during early stages of research such as surgical staff, surgical procedure, and the challenges of working with live tissue. One such example is the da Vinci Research Kit (dVRK) which is used by over 40 robotics research groups and represents an open-sourced version of the da Vinci Surgical System. Despite dVRK being available for nearly a decade and the ideal candidate for translating research to practice on over 5,000 da Vinci Systems used in hospitals around the world, not one live surgery has been conducted with it. In this paper, we address the challenges, considerations, and solutions for translating surgical robotic research from bench-to-bedside. This is explained from the perspective of a remote telesurgery scenario where motion scaling solutions previously experimented in a lab setting are translated to a live pig surgery. This study presents results from the first ever use of a dVRK in a live animal and discusses how the surgical robotics community can approach translating their research to practice.
Bimanual Regrasping for Suture Needles using Reinforcement Learning for Rapid Motion Planning
Nov 09, 2020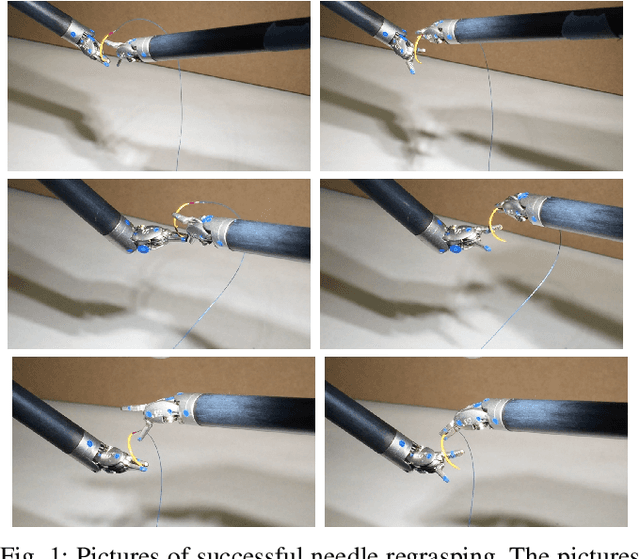

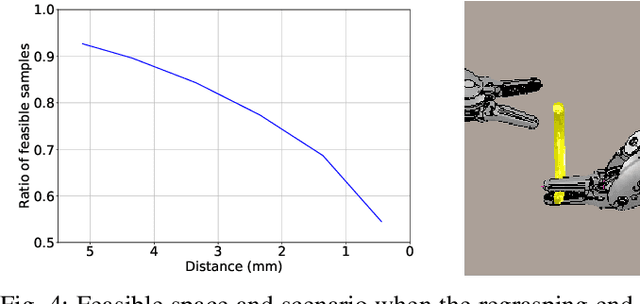
Abstract:Regrasping a suture needle is an important process in suturing, and previous study has shown that it takes on average 7.4s before the needle is thrown again. To bring efficiency into suturing, prior work either designs a task-specific mechanism or guides the gripper toward some specific pick-up point for proper grasping of a needle. Yet, these methods are usually not deployable when the working space is changed. These prior efforts highlight the need for more efficient regrasping and more generalizability of a proposed method. Therefore, in this work, we present rapid trajectory generation for bimanual needle regrasping via reinforcement learning (RL). Demonstrations from a sampling-based motion planning algorithm is incorporated to speed up the learning. In addition, we propose the ego-centric state and action spaces for this bimanual planning problem, where the reference frames are on the end-effectors instead of some fixed frame. Thus, the learned policy can be directly applied to any robot configuration and even to different robot arms. Our experiments in simulation show that the success rate of a single pass is 97%, and the planning time is 0.0212s on average, which outperforms other widely used motion planning algorithms. For the real-world experiments, the success rate is 73.3% if the needle pose is reconstructed from an RGB image, with a planning time of 0.0846s and a run time of 5.1454s. If the needle pose is known beforehand, the success rate becomes 90.5%, with a planning time of 0.0807s and a run time of 2.8801s.
Autonomous Robotic Suction to Clear the Surgical Field for Hemostasis using Image-based Blood Flow Detection
Oct 16, 2020



Abstract:Autonomous robotic surgery has seen significant progression over the last decade with the aims of reducing surgeon fatigue, improving procedural consistency, and perhaps one day take over surgery itself. However, automation has not been applied to the critical surgical task of controlling tissue and blood vessel bleeding--known as hemostasis. The task of hemostasis covers a spectrum of bleeding sources and a range of blood velocity, trajectory, and volume. In an extreme case, an un-controlled blood vessel fills the surgical field with flowing blood. In this work, we present the first, automated solution for hemostasis through development of a novel probabilistic blood flow detection algorithm and a trajectory generation technique that guides autonomous suction tools towards pooling blood. The blood flow detection algorithm is tested in both simulated scenes and in a real-life trauma scenario involving a hemorrhage that occurred during thyroidectomy. The complete solution is tested in a physical lab setting with the da Vinci Research Kit (dVRK) and a simulated surgical cavity for blood to flow through. The results show that our automated solution has accurate detection, a fast reaction time, and effective removal of the flowing blood. Therefore, the proposed methods are powerful tools to clearing the surgical field which can be followed by either a surgeon or future robotic automation developments to close the vessel rupture.
SuPer: A Surgical Perception Framework for Endoscopic Tissue Manipulation with Surgical Robotics
Sep 11, 2019
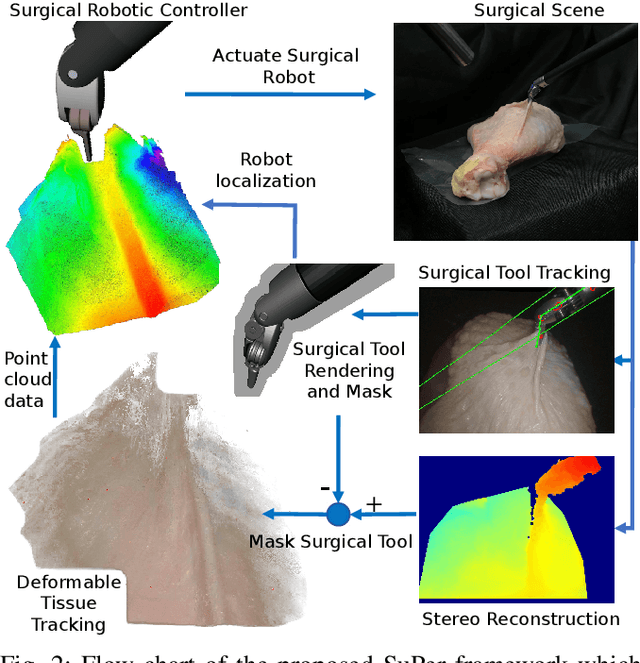


Abstract:Traditional control and task automation have been successfully demonstrated in a variety of structured, controlled environments through the use of highly specialized modeled robotic systems in conjunction with multiple sensors. However, application of autonomy in endoscopic surgery is very challenging, particularly in soft tissue work, due to the lack of high-quality images and the unpredictable, constantly deforming environment. In this work, we propose a novel surgical perception framework, SuPer, for surgical robotic control. This framework continuously collects 3D geometric information that allows for mapping of a deformable surgical field while tracking rigid instruments within the field. To achieve this, a model-based tracker is employed to localize the surgical tool with a kinematic prior in conjunction with a model-free tracker to reconstruct the deformable environment and provide an estimated point cloud as a mapping of the environment. The proposed framework was implemented on the da Vinci Surgical System in real-time with an end-effector controller where the target configurations are set and regulated through the framework. Our proposed framework successfully completed autonomous soft tissue manipulation tasks with high accuracy. The demonstration of this novel framework is promising for the future of surgical autonomy. In addition, we provide our dataset for further surgical research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge