E. Shelley Hwang
Breast density in MRI: an AI-based quantification and relationship to assessment in mammography
Apr 21, 2025Abstract:Mammographic breast density is a well-established risk factor for breast cancer. Recently there has been interest in breast MRI as an adjunct to mammography, as this modality provides an orthogonal and highly quantitative assessment of breast tissue. However, its 3D nature poses analytic challenges related to delineating and aggregating complex structures across slices. Here, we applied an in-house machine-learning algorithm to assess breast density on normal breasts in three MRI datasets. Breast density was consistent across different datasets (0.104 - 0.114). Analysis across different age groups also demonstrated strong consistency across datasets and confirmed a trend of decreasing density with age as reported in previous studies. MR breast density was correlated with mammographic breast density, although some notable differences suggest that certain breast density components are captured only on MRI. Future work will determine how to integrate MR breast density with current tools to improve future breast cancer risk prediction.
Practical Design and Benchmarking of Generative AI Applications for Surgical Billing and Coding
Jan 07, 2025



Abstract:Background: Healthcare has many manual processes that can benefit from automation and augmentation with Generative Artificial Intelligence (AI), the medical billing and coding process. However, current foundational Large Language Models (LLMs) perform poorly when tasked with generating accurate International Classification of Diseases, 10th edition, Clinical Modification (ICD-10-CM) and Current Procedural Terminology (CPT) codes. Additionally, there are many security and financial challenges in the application of generative AI to healthcare. We present a strategy for developing generative AI tools in healthcare, specifically for medical billing and coding, that balances accuracy, accessibility, and patient privacy. Methods: We fine tune the PHI-3 Mini and PHI-3 Medium LLMs using institutional data and compare the results against the PHI-3 base model, a PHI-3 RAG application, and GPT-4o. We use the post operative surgical report as input and the patients billing claim the associated ICD-10, CPT, and Modifier codes as the target result. Performance is measured by accuracy of code generation, proportion of invalid codes, and the fidelity of the billing claim format. Results: Both fine-tuned models performed better or as well as GPT-4o. The Phi-3 Medium fine-tuned model showed the best performance (ICD-10 Recall and Precision: 72%, 72%; CPT Recall and Precision: 77%, 79%; Modifier Recall and Precision: 63%, 64%). The Phi-3 Medium fine-tuned model only fabricated 1% of ICD-10 codes and 0.6% of CPT codes generated. Conclusions: Our study shows that a small model that is fine-tuned on domain-specific data for specific tasks using a simple set of open-source tools and minimal technological and monetary requirements performs as well as the larger contemporary consumer models.
Deep learning analysis of breast MRIs for prediction of occult invasive disease in ductal carcinoma in situ
Nov 28, 2017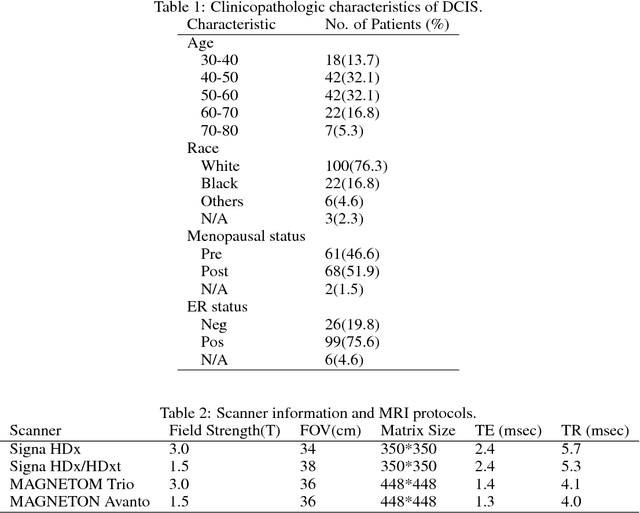
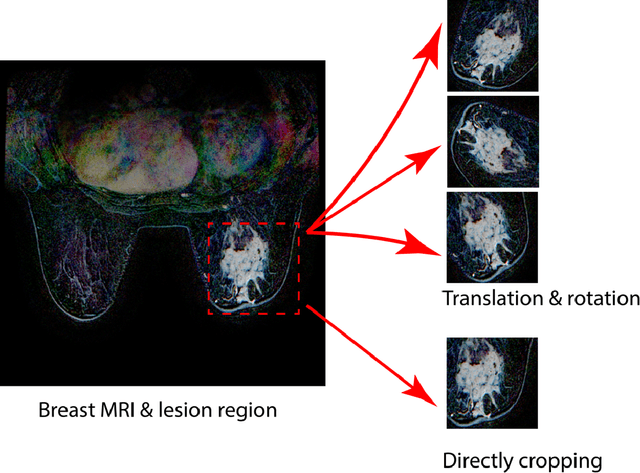
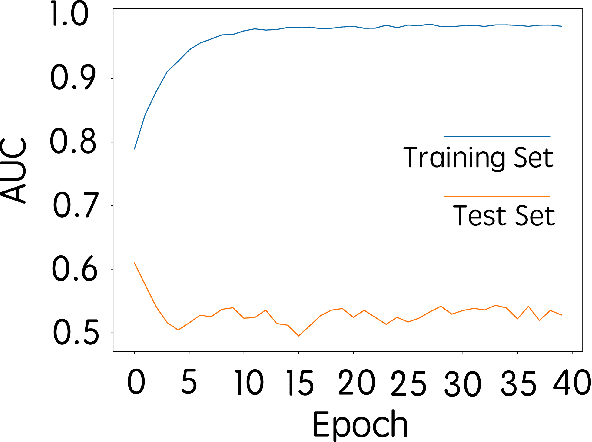
Abstract:Purpose: To determine whether deep learning-based algorithms applied to breast MR images can aid in the prediction of occult invasive disease following the di- agnosis of ductal carcinoma in situ (DCIS) by core needle biopsy. Material and Methods: In this institutional review board-approved study, we analyzed dynamic contrast-enhanced fat-saturated T1-weighted MRI sequences of 131 patients at our institution with a core needle biopsy-confirmed diagnosis of DCIS. The patients had no preoperative therapy before breast MRI and no prior history of breast cancer. We explored two different deep learning approaches to predict whether there was a hidden (occult) invasive component in the analyzed tumors that was ultimately detected at surgical excision. In the first approach, we adopted the transfer learning strategy, in which a network pre-trained on a large dataset of natural images is fine-tuned with our DCIS images. Specifically, we used the GoogleNet model pre-trained on the ImageNet dataset. In the second approach, we used a pre-trained network to extract deep features, and a support vector machine (SVM) that utilizes these features to predict the upstaging of the DCIS. We used 10-fold cross validation and the area under the ROC curve (AUC) to estimate the performance of the predictive models. Results: The best classification performance was obtained using the deep features approach with GoogleNet model pre-trained on ImageNet as the feature extractor and a polynomial kernel SVM used as the classifier (AUC = 0.70, 95% CI: 0.58- 0.79). For the transfer learning based approach, the highest AUC obtained was 0.53 (95% CI: 0.41-0.62). Conclusion: Convolutional neural networks could potentially be used to identify occult invasive disease in patients diagnosed with DCIS at the initial core needle biopsy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge