Ozanan Meireles
Practical Design and Benchmarking of Generative AI Applications for Surgical Billing and Coding
Jan 07, 2025



Abstract:Background: Healthcare has many manual processes that can benefit from automation and augmentation with Generative Artificial Intelligence (AI), the medical billing and coding process. However, current foundational Large Language Models (LLMs) perform poorly when tasked with generating accurate International Classification of Diseases, 10th edition, Clinical Modification (ICD-10-CM) and Current Procedural Terminology (CPT) codes. Additionally, there are many security and financial challenges in the application of generative AI to healthcare. We present a strategy for developing generative AI tools in healthcare, specifically for medical billing and coding, that balances accuracy, accessibility, and patient privacy. Methods: We fine tune the PHI-3 Mini and PHI-3 Medium LLMs using institutional data and compare the results against the PHI-3 base model, a PHI-3 RAG application, and GPT-4o. We use the post operative surgical report as input and the patients billing claim the associated ICD-10, CPT, and Modifier codes as the target result. Performance is measured by accuracy of code generation, proportion of invalid codes, and the fidelity of the billing claim format. Results: Both fine-tuned models performed better or as well as GPT-4o. The Phi-3 Medium fine-tuned model showed the best performance (ICD-10 Recall and Precision: 72%, 72%; CPT Recall and Precision: 77%, 79%; Modifier Recall and Precision: 63%, 64%). The Phi-3 Medium fine-tuned model only fabricated 1% of ICD-10 codes and 0.6% of CPT codes generated. Conclusions: Our study shows that a small model that is fine-tuned on domain-specific data for specific tasks using a simple set of open-source tools and minimal technological and monetary requirements performs as well as the larger contemporary consumer models.
Hypergraph-Transformer (HGT) for Interactive Event Prediction in Laparoscopic and Robotic Surgery
Feb 03, 2024



Abstract:Understanding and anticipating intraoperative events and actions is critical for intraoperative assistance and decision-making during minimally invasive surgery. Automated prediction of events, actions, and the following consequences is addressed through various computational approaches with the objective of augmenting surgeons' perception and decision-making capabilities. We propose a predictive neural network that is capable of understanding and predicting critical interactive aspects of surgical workflow from intra-abdominal video, while flexibly leveraging surgical knowledge graphs. The approach incorporates a hypergraph-transformer (HGT) structure that encodes expert knowledge into the network design and predicts the hidden embedding of the graph. We verify our approach on established surgical datasets and applications, including the detection and prediction of action triplets, and the achievement of the Critical View of Safety (CVS). Moreover, we address specific, safety-related tasks, such as predicting the clipping of cystic duct or artery without prior achievement of the CVS. Our results demonstrate the superiority of our approach compared to unstructured alternatives.
SUrgical PRediction GAN for Events Anticipation
May 10, 2021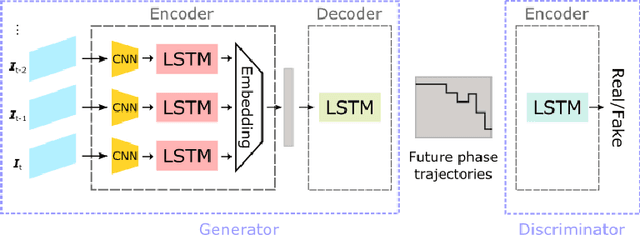
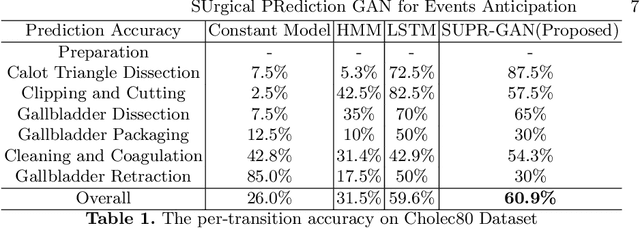
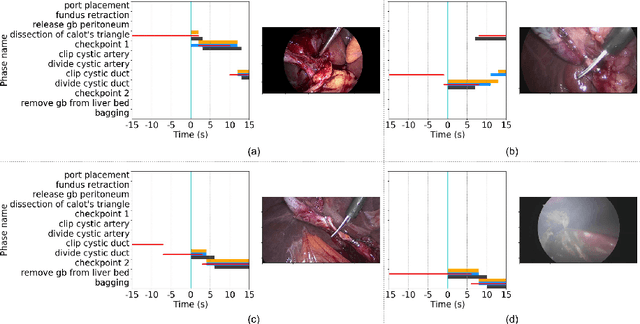
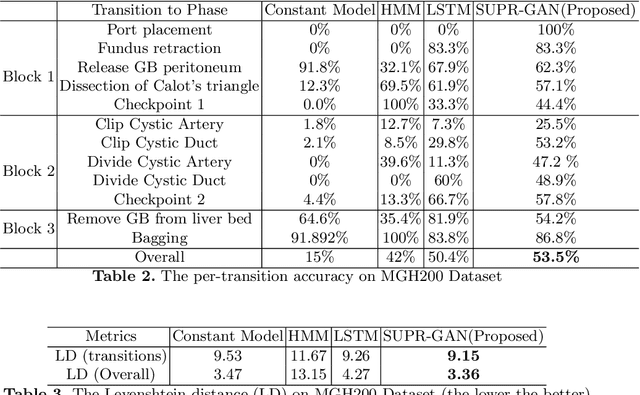
Abstract:Comprehension of surgical workflow is the foundation upon which computers build the understanding of surgery. In this work, we moved beyond just the identification of surgical phases to predict future surgical phases and the transitions between them. We used a novel GAN formulation that sampled the future surgical phases trajectory conditioned, on past laparoscopic video frames, and compared it to state-of-the-art approaches for surgical video analysis and alternative prediction methods. We demonstrated its effectiveness in inferring and predicting the progress of laparoscopic cholecystectomy videos. We quantified the horizon-accuracy trade-off and explored average performance as well as the performance on the more difficult, and clinically important, transitions between phases. Lastly, we surveyed surgeons to evaluate the plausibility of these predicted trajectories.
Aggregating Long-Term Context for Learning Surgical Workflows
Sep 11, 2020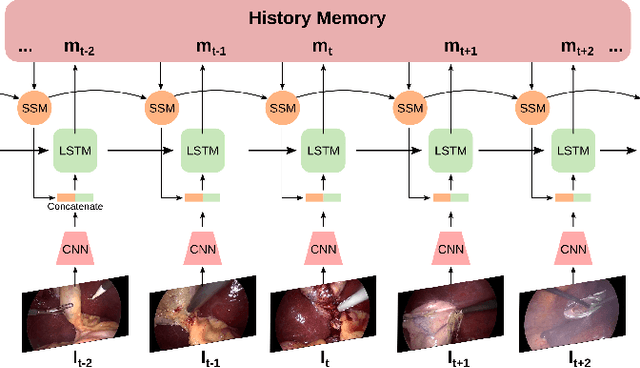
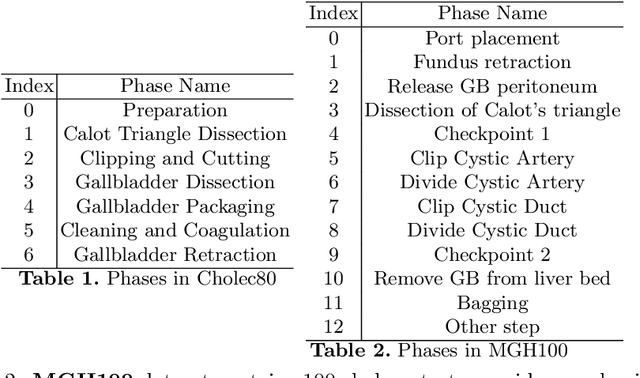
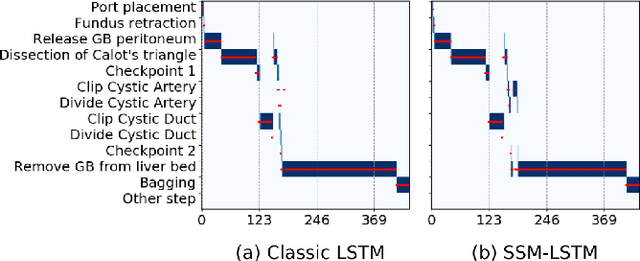

Abstract:Analyzing surgical workflow is crucial for computers to understand surgeries. Deep learning techniques have recently been widely applied to recognize surgical workflows. Many of the existing temporal neural network models are limited in their capability to handle long-term dependencies in the data, instead of relying upon strong performance of the underlying per-frame visual models. We propose a new temporal network structure that leverages task-specific network representation to collect long-term sufficient statistics that are propagated by a sufficient statistics model (SSM). We leverage our approach within an LSTM back-bone for the task of surgical phase recognition and explore several choices for propagated statistics. We demonstrate superior results over existing state-of-the-art segmentation and novel segmentation techniques, on two laparoscopic cholecystectomy datasets: the already published Cholec80dataset and MGH100, a novel dataset with more challenging, yet clinically meaningful, segment labels.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge