Damien Garcia
CREATIS
Ultrasound Image Generation using Latent Diffusion Models
Feb 12, 2025Abstract:Diffusion models for image generation have been a subject of increasing interest due to their ability to generate diverse, high-quality images. Image generation has immense potential in medical imaging because open-source medical images are difficult to obtain compared to natural images, especially for rare conditions. The generated images can be used later to train classification and segmentation models. In this paper, we propose simulating realistic ultrasound (US) images by successive fine-tuning of large diffusion models on different publicly available databases. To do so, we fine-tuned Stable Diffusion, a state-of-the-art latent diffusion model, on BUSI (Breast US Images) an ultrasound breast image dataset. We successfully generated high-quality US images of the breast using simple prompts that specify the organ and pathology, which appeared realistic to three experienced US scientists and a US radiologist. Additionally, we provided user control by conditioning the model with segmentations through ControlNet. We will release the source code at http://code.sonography.ai/ to allow fast US image generation to the scientific community.
Boosting Cardiac Color Doppler Frame Rates with Deep Learning
Mar 28, 2024Abstract:Color Doppler echocardiography enables visualization of blood flow within the heart. However, the limited frame rate impedes the quantitative assessment of blood velocity throughout the cardiac cycle, thereby compromising a comprehensive analysis of ventricular filling. Concurrently, deep learning is demonstrating promising outcomes in post-processing of echocardiographic data for various applications. This work explores the use of deep learning models for intracardiac Doppler velocity estimation from a reduced number of filtered I/Q signals. We used a supervised learning approach by simulating patient-based cardiac color Doppler acquisitions and proposed data augmentation strategies to enlarge the training dataset. We implemented architectures based on convolutional neural networks. In particular, we focused on comparing the U-Net model and the recent ConvNeXt models, alongside assessing real-valued versus complex-valued representations. We found that both models outperformed the state-of-the-art autocorrelator method, effectively mitigating aliasing and noise. We did not observe significant differences between the use of real and complex data. Finally, we validated the models on in vitro and in vivo experiments. All models produced quantitatively comparable results to the baseline and were more robust to noise. ConvNeXt emerged as the sole model to achieve high-quality results on in vivo aliased samples. These results demonstrate the interest of supervised deep learning methods for Doppler velocity estimation from a reduced number of acquisitions.
Physics-Guided Neural Networks for Intraventricular Vector Flow Mapping
Mar 19, 2024



Abstract:Intraventricular vector flow mapping (iVFM) seeks to enhance and quantify color Doppler in cardiac imaging. In this study, we propose novel alternatives to the traditional iVFM optimization scheme by utilizing physics-informed neural networks (PINNs) and a physics-guided nnU-Net-based supervised approach. Through rigorous evaluation on simulated color Doppler images derived from a patient-specific computational fluid dynamics model and in vivo Doppler acquisitions, both approaches demonstrate comparable reconstruction performance to the original iVFM algorithm. The efficiency of PINNs is boosted through dual-stage optimization and pre-optimized weights. On the other hand, the nnU-Net method excels in generalizability and real time capabilities. Notably, nnU-Net shows superior robustness on sparse and truncated Doppler data while maintaining independence from explicit boundary conditions. Overall, our results highlight the effectiveness of these methods in reconstructing intraventricular vector blood flow. The study also suggests potential applications of PINNs in ultrafast color Doppler imaging and the incorporation of fluid dynamics equations to derive biomarkers for cardiovascular diseases based on blood flow.
Phase Unwrapping of Color Doppler Echocardiography using Deep Learning
Jul 05, 2023



Abstract:Color Doppler echocardiography is a widely used non-invasive imaging modality that provides real-time information about the intracardiac blood flow. In an apical long-axis view of the left ventricle, color Doppler is subject to phase wrapping, or aliasing, especially during cardiac filling and ejection. When setting up quantitative methods based on color Doppler, it is necessary to correct this wrapping artifact. We developed an unfolded primal-dual network to unwrap (dealias) color Doppler echocardiographic images and compared its effectiveness against two state-of-the-art segmentation approaches based on nnU-Net and transformer models. We trained and evaluated the performance of each method on an in-house dataset and found that the nnU-Net-based method provided the best dealiased results, followed by the primal-dual approach and the transformer-based technique. Noteworthy, the primal-dual network, which had significantly fewer trainable parameters, performed competitively with respect to the other two methods, demonstrating the high potential of deep unfolding methods. Our results suggest that deep learning-based methods can effectively remove aliasing artifacts in color Doppler echocardiographic images, outperforming DeAN, a state-of-the-art semi-automatic technique. Overall, our results show that deep learning-based methods have the potential to effectively preprocess color Doppler images for downstream quantitative analysis.
Ultrafast Cardiac Imaging Using Deep Learning For Speckle-Tracking Echocardiography
Jun 25, 2023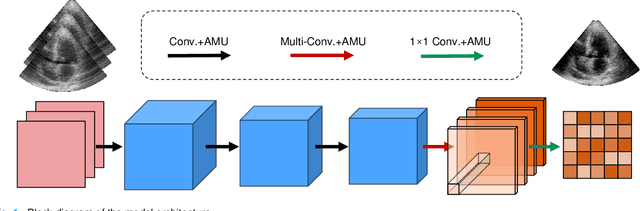
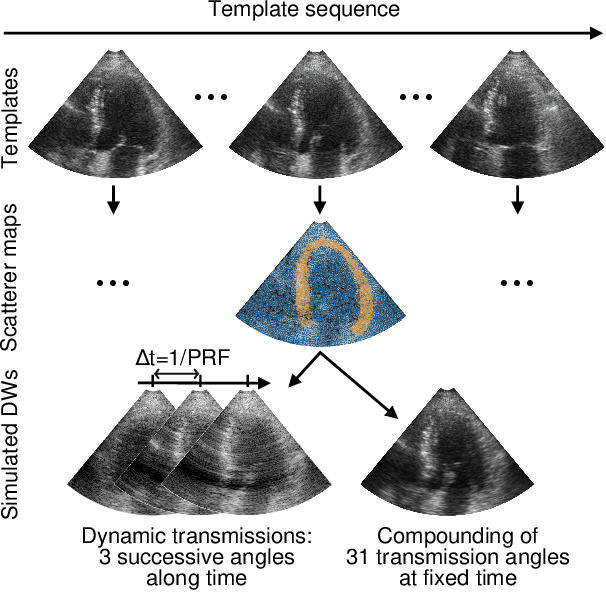
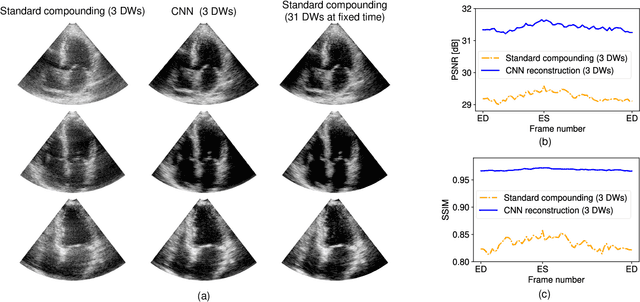
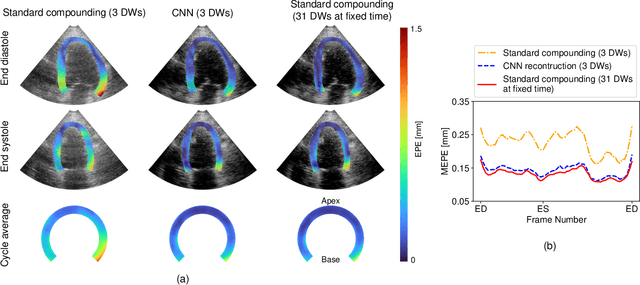
Abstract:High-quality ultrafast ultrasound imaging is based on coherent compounding from multiple transmissions of plane waves (PW) or diverging waves (DW). However, compounding results in reduced frame rate, as well as destructive interferences from high-velocity tissue motion if motion compensation (MoCo) is not considered. While many studies have recently shown the interest of deep learning for the reconstruction of high-quality static images from PW or DW, its ability to achieve such performance while maintaining the capability of tracking cardiac motion has yet to be assessed. In this paper, we addressed such issue by deploying a complex-weighted convolutional neural network (CNN) for image reconstruction and a state-of-the-art speckle tracking method. The evaluation of this approach was first performed by designing an adapted simulation framework, which provides specific reference data, i.e. high quality, motion artifact-free cardiac images. The obtained results showed that, while using only three DWs as input, the CNN-based approach yielded an image quality and a motion accuracy equivalent to those obtained by compounding 31 DWs free of motion artifacts. The performance was then further evaluated on non-simulated, experimental in vitro data, using a spinning disk phantom. This experiment demonstrated that our approach yielded high-quality image reconstruction and motion estimation, under a large range of velocities and outperforms a state-of-the-art MoCo-based approach at high velocities. Our method was finally assessed on in vivo datasets and showed consistent improvement in image quality and motion estimation compared to standard compounding. This demonstrates the feasibility and effectiveness of deep learning reconstruction for ultrafast speckle-tracking echocardiography.
Extraction of volumetric indices from echocardiography: which deep learning solution for clinical use?
May 08, 2023Abstract:Deep learning-based methods have spearheaded the automatic analysis of echocardiographic images, taking advantage of the publication of multiple open access datasets annotated by experts (CAMUS being one of the largest public databases). However, these models are still considered unreliable by clinicians due to unresolved issues concerning i) the temporal consistency of their predictions, and ii) their ability to generalize across datasets. In this context, we propose a comprehensive comparison between the current best performing methods in medical/echocardiographic image segmentation, with a particular focus on temporal consistency and cross-dataset aspects. We introduce a new private dataset, named CARDINAL, of apical two-chamber and apical four-chamber sequences, with reference segmentation over the full cardiac cycle. We show that the proposed 3D nnU-Net outperforms alternative 2D and recurrent segmentation methods. We also report that the best models trained on CARDINAL, when tested on CAMUS without any fine-tuning, still manage to perform competitively with respect to prior methods. Overall, the experimental results suggest that with sufficient training data, 3D nnU-Net could become the first automated tool to finally meet the standards of an everyday clinical device.
SIMUS: an open-source simulator for ultrasound imaging. Part I: theory & examples
Feb 04, 2021



Abstract:Computational ultrasound imaging has become a well-established methodology in the ultrasound community. Simulations of ultrasound sequences and images allow the study of innovative techniques in terms of emission strategy, beamforming and probe design. There is a wide spectrum of software dedicated to ultrasound imaging, each having its specificities in its applications and in the numerical method. I describe in this two-part paper a new ultrasound simulator (SIMUS) for Matlab, which belongs to the Matlab UltraSound Toolbox (MUST). The SIMUS software is based on far-field and paraxial approximations. It simulates acoustic pressure fields and radiofrequency RF signals for uniform linear or convex probes. SIMUS is an open-source software whose features are 1) rapidity, 2) ease of use, 3) frequency domain, 4) pedagogy. The main goal was to offer a comprehensive turnkey tool, along with a detailed theory for pedagogical and research purposes. This first part of the paper describes in detail the underlying linear theory of SIMUS and provides examples of simulated acoustic fields and ultrasound images. The second part is devoted to the comparison of SIMUS with popular software: Field II, k-Wave, and the Verasonics simulator. The Matlab open codes for the simulator SIMUS are distributed under the terms of the GNU Lesser General Public License, and can be downloaded from https://www.biomecardio.com/MUST.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge