Clement C. Tham
A graph neural network-based multispectral-view learning model for diabetic macular ischemia detection from color fundus photographs
Feb 25, 2025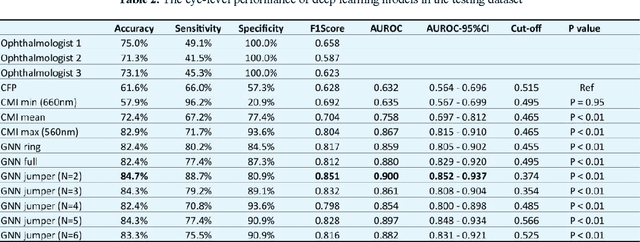
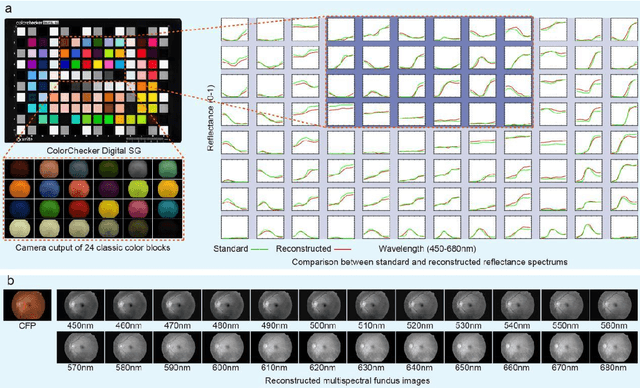
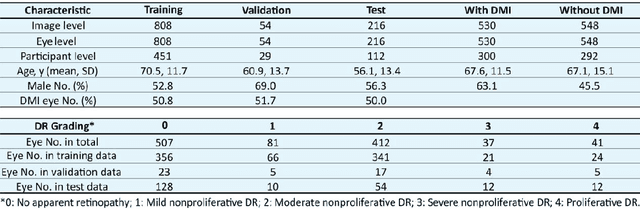
Abstract:Diabetic macular ischemia (DMI), marked by the loss of retinal capillaries in the macular area, contributes to vision impairment in patients with diabetes. Although color fundus photographs (CFPs), combined with artificial intelligence (AI), have been extensively applied in detecting various eye diseases, including diabetic retinopathy (DR), their applications in detecting DMI remain unexplored, partly due to skepticism among ophthalmologists regarding its feasibility. In this study, we propose a graph neural network-based multispectral view learning (GNN-MSVL) model designed to detect DMI from CFPs. The model leverages higher spectral resolution to capture subtle changes in fundus reflectance caused by ischemic tissue, enhancing sensitivity to DMI-related features. The proposed approach begins with computational multispectral imaging (CMI) to reconstruct 24-wavelength multispectral fundus images from CFPs. ResNeXt101 is employed as the backbone for multi-view learning to extract features from the reconstructed images. Additionally, a GNN with a customized jumper connection strategy is designed to enhance cross-spectral relationships, facilitating comprehensive and efficient multispectral view learning. The study included a total of 1,078 macula-centered CFPs from 1,078 eyes of 592 patients with diabetes, of which 530 CFPs from 530 eyes of 300 patients were diagnosed with DMI. The model achieved an accuracy of 84.7 percent and an area under the receiver operating characteristic curve (AUROC) of 0.900 (95 percent CI: 0.852-0.937) on eye-level, outperforming both the baseline model trained from CFPs and human experts (p-values less than 0.01). These findings suggest that AI-based CFP analysis holds promise for detecting DMI, contributing to its early and low-cost screening.
Unifying Structure Analysis and Surrogate-driven Function Regression for Glaucoma OCT Image Screening
Jul 26, 2019


Abstract:Optical Coherence Tomography (OCT) imaging plays an important role in glaucoma diagnosis in clinical practice. Early detection and timely treatment can prevent glaucoma patients from permanent vision loss. However, only a dearth of automated methods has been developed based on OCT images for glaucoma study. In this paper, we present a novel framework to effectively classify glaucoma OCT images from normal ones. A semi-supervised learning strategy with smoothness assumption is applied for surrogate assignment of missing function regression labels. Besides, the proposed multi-task learning network is capable of exploring the structure and function relationship from the OCT image and visual field measurement simultaneously, which contributes to classification performance boosting. Essentially, we are the first to unify the structure analysis and function regression for glaucoma screening. It is also worth noting that we build the largest glaucoma OCT image dataset involving 4877 volumes to develop and evaluate the proposed method. Extensive experiments demonstrate that our framework outperforms the baseline methods and two glaucoma experts by a large margin, achieving 93.2%, 93.2% and 97.8% on accuracy, F1 score and AUC, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge