Qinghua He
A graph neural network-based multispectral-view learning model for diabetic macular ischemia detection from color fundus photographs
Feb 25, 2025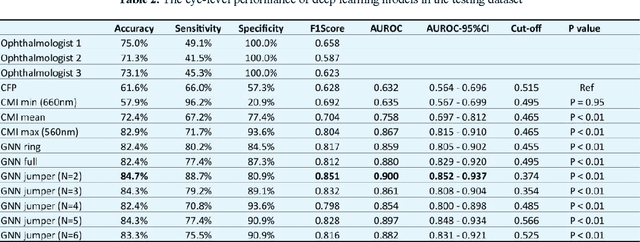
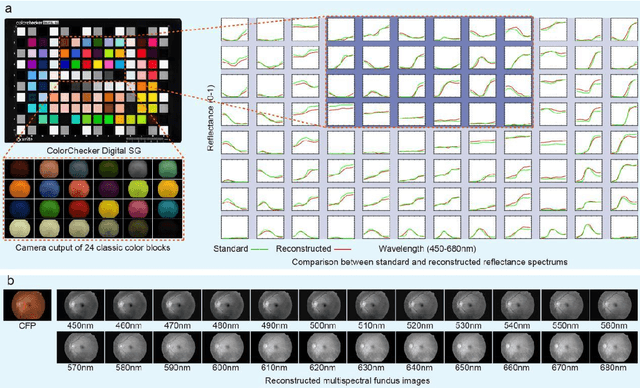
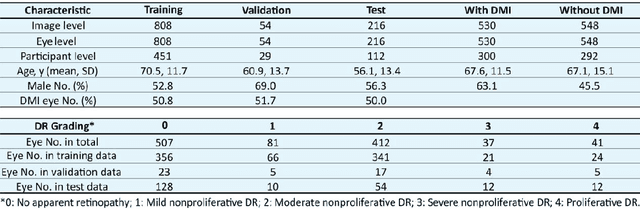
Abstract:Diabetic macular ischemia (DMI), marked by the loss of retinal capillaries in the macular area, contributes to vision impairment in patients with diabetes. Although color fundus photographs (CFPs), combined with artificial intelligence (AI), have been extensively applied in detecting various eye diseases, including diabetic retinopathy (DR), their applications in detecting DMI remain unexplored, partly due to skepticism among ophthalmologists regarding its feasibility. In this study, we propose a graph neural network-based multispectral view learning (GNN-MSVL) model designed to detect DMI from CFPs. The model leverages higher spectral resolution to capture subtle changes in fundus reflectance caused by ischemic tissue, enhancing sensitivity to DMI-related features. The proposed approach begins with computational multispectral imaging (CMI) to reconstruct 24-wavelength multispectral fundus images from CFPs. ResNeXt101 is employed as the backbone for multi-view learning to extract features from the reconstructed images. Additionally, a GNN with a customized jumper connection strategy is designed to enhance cross-spectral relationships, facilitating comprehensive and efficient multispectral view learning. The study included a total of 1,078 macula-centered CFPs from 1,078 eyes of 592 patients with diabetes, of which 530 CFPs from 530 eyes of 300 patients were diagnosed with DMI. The model achieved an accuracy of 84.7 percent and an area under the receiver operating characteristic curve (AUROC) of 0.900 (95 percent CI: 0.852-0.937) on eye-level, outperforming both the baseline model trained from CFPs and human experts (p-values less than 0.01). These findings suggest that AI-based CFP analysis holds promise for detecting DMI, contributing to its early and low-cost screening.
Choroidal thinning assessment through facial video analysis
Jan 29, 2024Abstract:Different features of skin are associated with various medical conditions and provide opportunities to evaluate and monitor body health. This study created a strategy to assess choroidal thinning through the video analysis of facial skin. Videos capturing the entire facial skin were collected from 48 participants with age-related macular degeneration (AMD) and 12 healthy individuals. These facial videos were analyzed using video-based trans-angiosomes imaging photoplethysmography (TaiPPG) to generate facial imaging biomarkers that were correlated with choroidal thickness (CT) measurements. The CT of all patients was determined using swept-source optical coherence tomography (SS-OCT). The results revealed the relationship between relative blood pulsation amplitude (BPA) in three typical facial angiosomes (cheek, side-forehead and mid-forehead) and the average macular CT (r = 0.48, p < 0.001; r = -0.56, p < 0.001; r = -0.40, p < 0.01). When considering a diagnostic threshold of 200{\mu}m, the newly developed facial video analysis tool effectively distinguished between cases of choroidal thinning and normal cases, yielding areas under the curve of 0.75, 0.79 and 0.69. These findings shed light on the connection between choroidal blood flow and facial skin hemodynamics, which suggests the potential for predicting vascular diseases through widely accessible skin imaging data.
Augmented smartphone bilirubinometer enabled by a mobile app that turns smartphone into multispectral imager
Mar 04, 2023


Abstract:We present the development of SpeCamX, a mobile application that transforms any unmodified smartphone into a powerful multispectral imager capable of capturing multispectral information. Our application includes an augmented bilirubinometer, enabling accurate prediction of blood bilirubin levels (BBL). In a clinical study involving 320 patients with liver diseases, we used SpeCamX to image the bulbar conjunctiva region, and we employed a hybrid machine learning prediction model to predict BBL. We observed a high correlation with blood test results, demonstrating the efficacy of our approach. Furthermore, we compared our method, which uses spectrally augmented learning (SAL), with traditional learning based on RGB photographs (RGBL), and our results clearly indicate that SpeCamX outperforms RGBL in terms of prediction accuracy, efficiency, and stability. This study highlights the potential of SpeCamX to improve the prediction of bio-chromophores, and its ability to transform an ordinary smartphone into a powerful medical tool without the need for additional investments or expertise. This makes it suitable for widespread use, particularly in areas where medical resources are scarce.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge