Christian T. Farrar
Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, USA
Dynamic and Rapid Deep Synthesis of Molecular MRI Signals
May 30, 2023Abstract:Model-driven analysis of biophysical phenomena is gaining increased attention and utility for medical imaging applications. In magnetic resonance imaging (MRI), the availability of well-established models for describing the relations between the nuclear magnetization, tissue properties, and the externally applied magnetic fields has enabled the prediction of image contrast and served as a powerful tool for designing the imaging protocols that are now routinely used in the clinic. Recently, various advanced imaging techniques have relied on these models for image reconstruction, quantitative tissue parameter extraction, and automatic optimization of acquisition protocols. In molecular MRI, however, the increased complexity of the imaging scenario, where the signals from various chemical compounds and multiple proton pools must be accounted for, results in exceedingly long model simulation times, severely hindering the progress of this approach and its dissemination for various clinical applications. Here, we show that a deep-learning-based system can capture the nonlinear relations embedded in the molecular MRI Bloch-McConnell model, enabling a rapid and accurate generation of biologically realistic synthetic data. The applicability of this simulated data for in-silico, in-vitro, and in-vivo imaging applications is then demonstrated for chemical exchange saturation transfer (CEST) and semisolid macromolecule magnetization transfer (MT) analysis and quantification. The proposed approach yielded 78%-99% acceleration in data synthesis time while retaining excellent agreement with the ground truth (Pearson's r$>$0.99, p$<$0.0001, normalized root mean square error $<$3%).
Accelerated and Quantitative 3D Semisolid MT/CEST Imaging using a Generative Adversarial Network (GAN-CEST)
Jul 22, 2022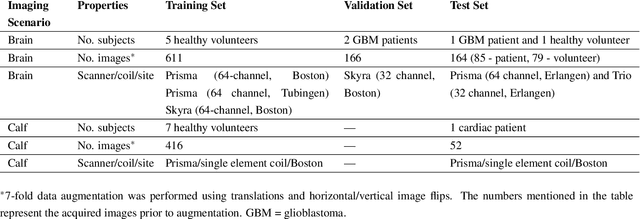
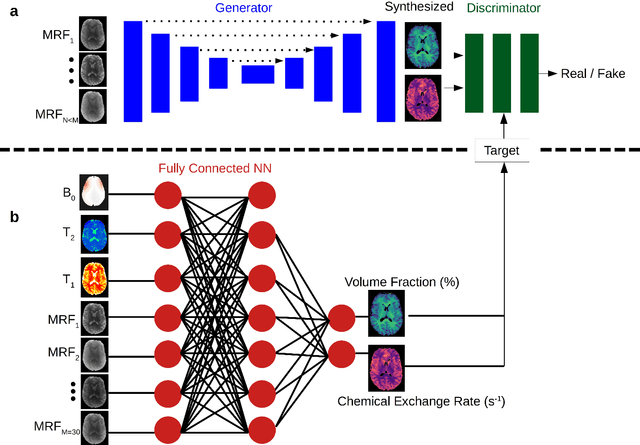
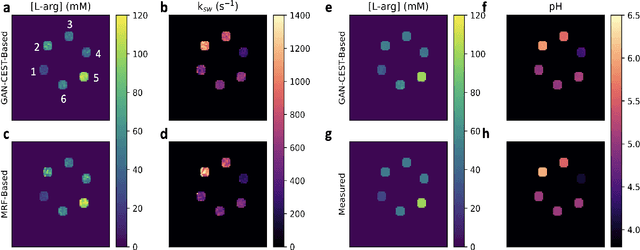
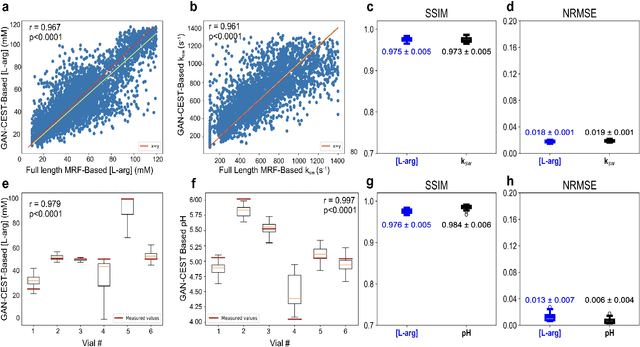
Abstract:Purpose: To substantially shorten the acquisition time required for quantitative 3D chemical exchange saturation transfer (CEST) and semisolid magnetization transfer (MT) imaging and allow for rapid chemical exchange parameter map reconstruction. Methods: Three-dimensional CEST and MT magnetic resonance fingerprinting (MRF) datasets of L-arginine phantoms, whole-brains, and calf muscles from healthy volunteers, cancer patients, and cardiac patients were acquired using 3T clinical scanners at 3 different sites, using 3 different scanner models and coils. A generative adversarial network supervised framework (GAN-CEST) was then designed and trained to learn the mapping from a reduced input data space to the quantitative exchange parameter space, while preserving perceptual and quantitative content. Results: The GAN-CEST 3D acquisition time was 42-52 seconds, 70% shorter than CEST-MRF. The quantitative reconstruction of the entire brain took 0.8 seconds. An excellent agreement was observed between the ground truth and GAN-based L-arginine concentration and pH values (Pearson's r > 0.97, NRMSE < 1.5%). GAN-CEST images from a brain-tumor subject yielded a semi-solid volume fraction and exchange rate NRMSE of 3.8$\pm$1.3% and 4.6$\pm$1.3%, respectively, and SSIM of 96.3$\pm$1.6% and 95.0$\pm$2.4%, respectively. The mapping of the calf-muscle exchange parameters in a cardiac patient, yielded NRMSE < 7% and SSIM > 94% for the semi-solid exchange parameters. In regions with large susceptibility artifacts, GAN-CEST has demonstrated improved performance and reduced noise compared to MRF. Conclusion: GAN-CEST can substantially reduce the acquisition time for quantitative semisolid MT/CEST mapping, while retaining performance even when facing pathologies and scanner models that were not available during training.
Development of a Clinical Chemical Exchange Saturation Transfer MR fingerprinting (CEST-MRF) Pulse Sequence and Reconstruction for Brain Tumor Quantification
Aug 18, 2021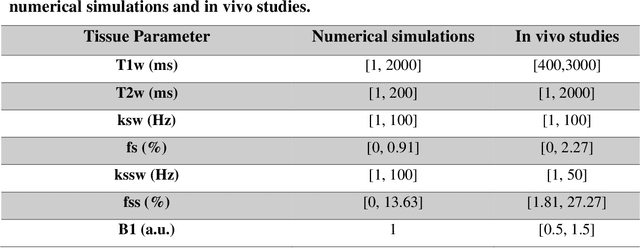



Abstract:Purpose: To develop a clinical chemical exchange saturation transfer magnetic resonance fingerprinting (CEST-MRF) pulse sequence and reconstruction method. Methods: The CEST-MRF pulse sequence was modified to conform to hardware limits on clinical scanners while keeping scan time $\leqslant$ 2 minutes. The measured data was reconstructed using a deep reconstruction network (DRONE) to yield the water relaxation and chemical exchange parameters. The feasibility of the 6 parameter DRONE reconstruction was tested in simulations in a digital brain phantom. A healthy subject was scanned with the CEST-MRF sequence and a conventional MRF sequence for comparison. The reproducibility was assessed via test-retest experiments and the concordance correlation coefficient (CCC) calculated for white matter (WM) and grey matter (GM). The clinical utility of CEST-MRF was demonstrated in a brain metastasis patient in comparison to standard clinical imaging sequences. The tumor was segmented into edema, solid core and necrotic core regions and the CEST-MRF values compared to the contra-lateral side. Results: The 6 parameter DRONE reconstruction of the digital phantom yielded a mean absolute error of $\leqslant$ 6% for all parameters. The CEST-MRF parameters were in good agreement with those from a conventional MRF sequence and previous studies in the literature. The mean CCC for all 6 parameters was 0.79$\pm$0.02 in WM and 0.63$\pm$0.03 in GM. The CEST-MRF values in nearly all tumor regions were significantly different (p=0.001) from each other and the contra-lateral side. Conclusion: The clinical CEST-MRF sequence provides a method for fast simultaneous quantification of multiple tissue parameters in pathologies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge