Ouri Cohen
Development of a Clinical Chemical Exchange Saturation Transfer MR fingerprinting (CEST-MRF) Pulse Sequence and Reconstruction for Brain Tumor Quantification
Aug 18, 2021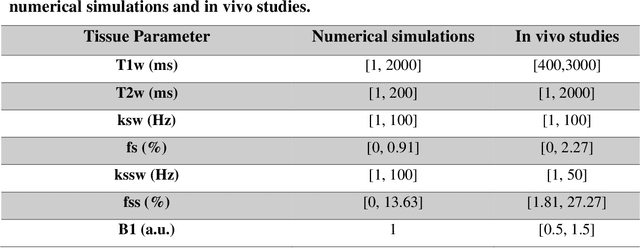



Abstract:Purpose: To develop a clinical chemical exchange saturation transfer magnetic resonance fingerprinting (CEST-MRF) pulse sequence and reconstruction method. Methods: The CEST-MRF pulse sequence was modified to conform to hardware limits on clinical scanners while keeping scan time $\leqslant$ 2 minutes. The measured data was reconstructed using a deep reconstruction network (DRONE) to yield the water relaxation and chemical exchange parameters. The feasibility of the 6 parameter DRONE reconstruction was tested in simulations in a digital brain phantom. A healthy subject was scanned with the CEST-MRF sequence and a conventional MRF sequence for comparison. The reproducibility was assessed via test-retest experiments and the concordance correlation coefficient (CCC) calculated for white matter (WM) and grey matter (GM). The clinical utility of CEST-MRF was demonstrated in a brain metastasis patient in comparison to standard clinical imaging sequences. The tumor was segmented into edema, solid core and necrotic core regions and the CEST-MRF values compared to the contra-lateral side. Results: The 6 parameter DRONE reconstruction of the digital phantom yielded a mean absolute error of $\leqslant$ 6% for all parameters. The CEST-MRF parameters were in good agreement with those from a conventional MRF sequence and previous studies in the literature. The mean CCC for all 6 parameters was 0.79$\pm$0.02 in WM and 0.63$\pm$0.03 in GM. The CEST-MRF values in nearly all tumor regions were significantly different (p=0.001) from each other and the contra-lateral side. Conclusion: The clinical CEST-MRF sequence provides a method for fast simultaneous quantification of multiple tissue parameters in pathologies.
MR fingerprinting Deep RecOnstruction NEtwork (DRONE)
Apr 24, 2018



Abstract:PURPOSE: Demonstrate a novel fast method for reconstruction of multi-dimensional MR Fingerprinting (MRF) data using Deep Learning methods. METHODS: A neural network (NN) is defined using the TensorFlow framework and trained on simulated MRF data computed using the Bloch equations. The accuracy of the NN reconstruction of noisy data is compared to conventional MRF template matching as a function of training data size, and quantified in a both simulated numerical brain phantom data and acquired data from the ISMRM/NIST phantom. The utility of the method is demonstrated in a healthy subject in vivo at 1.5 T. RESULTS: Network training required 10 minutes and once trained, data reconstruction required approximately 10 ms. Reconstruction of simulated brain data using the NN resulted in a root-mean-square error (RMSE) of 3.5 ms for T1 and 7.8 ms for T2. The RMSE for the NN trained on sparse dictionaries was approximately 6 fold lower for T1 and 2 fold lower for T2 than conventional MRF dot-product dictionary matching on the same dictionaries. Phantom measurements yielded good agreement (R2=0.99) between the T1 and T2 estimated by the NN and reference values from the ISMRM/NIST phantom. CONCLUSION: Reconstruction of MRF data with a NN is accurate, 300 fold faster and more robust to noise and undersampling than conventional MRF dictionary matching.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge