Chandra Sripada
An AI-Powered Research Assistant in the Lab: A Practical Guide for Text Analysis Through Iterative Collaboration with LLMs
May 14, 2025Abstract:Analyzing texts such as open-ended responses, headlines, or social media posts is a time- and labor-intensive process highly susceptible to bias. LLMs are promising tools for text analysis, using either a predefined (top-down) or a data-driven (bottom-up) taxonomy, without sacrificing quality. Here we present a step-by-step tutorial to efficiently develop, test, and apply taxonomies for analyzing unstructured data through an iterative and collaborative process between researchers and LLMs. Using personal goals provided by participants as an example, we demonstrate how to write prompts to review datasets and generate a taxonomy of life domains, evaluate and refine the taxonomy through prompt and direct modifications, test the taxonomy and assess intercoder agreements, and apply the taxonomy to categorize an entire dataset with high intercoder reliability. We discuss the possibilities and limitations of using LLMs for text analysis.
Image-on-Scalar Regression via Deep Neural Networks
Jun 17, 2020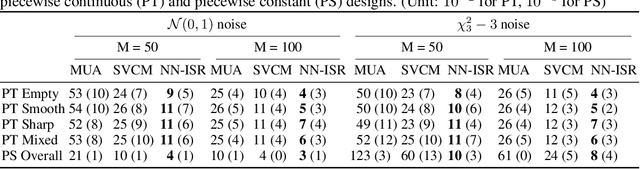
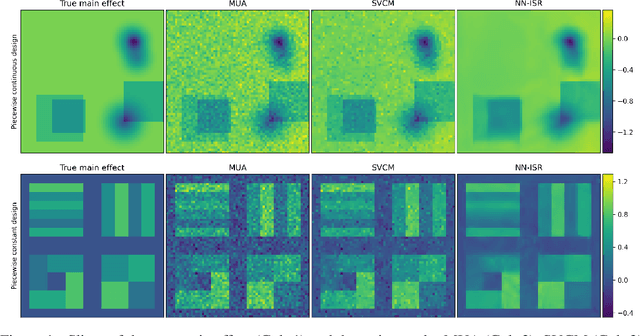
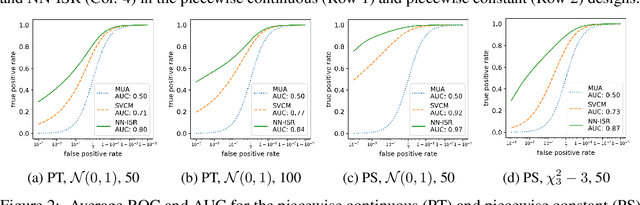
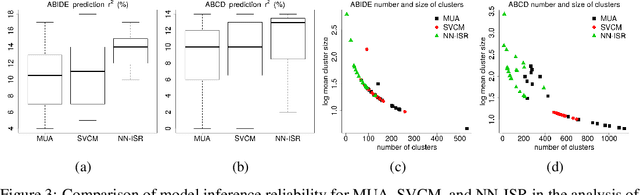
Abstract:A research topic of central interest in neuroimaging analysis is to study the associations between the massive imaging data and a set of covariates. This problem is challenging, due to the ultrahigh dimensionality, the high and heterogeneous level of noise, and the limited sample size of the imaging data. To address those challenges, we develop a novel image-on-scalar regression model, where the spatially-varying coefficients and the individual spatial effects are all constructed through deep neural networks (DNN). Compared with the existing solutions, our method is much more flexible in capturing the complex patterns among the brain signals, of which the noise level and the spatial smoothness appear to be heterogeneous across different brain regions. We develop a hybrid stochastic gradient descent estimation algorithm, and derive the asymptotic properties when the number of voxels grows much faster than the sample size. We show that the new method outperforms the existing ones through both extensive simulations and two neuroimaging data examples.
A Domain Guided CNN Architecture for Predicting Age from Structural Brain Images
Aug 11, 2018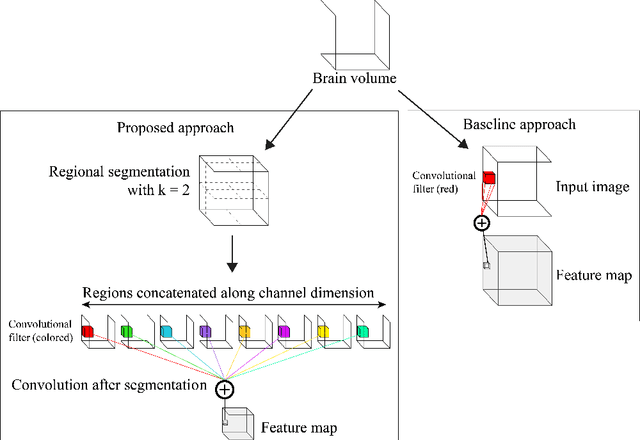
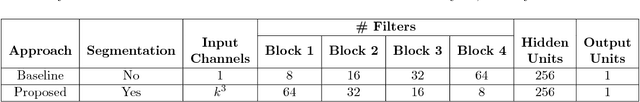
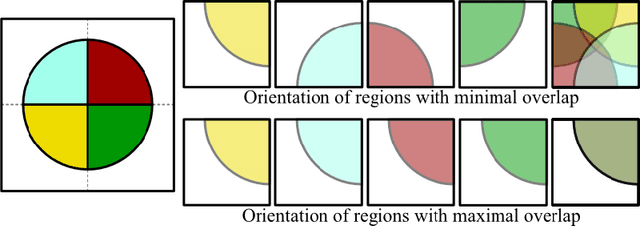
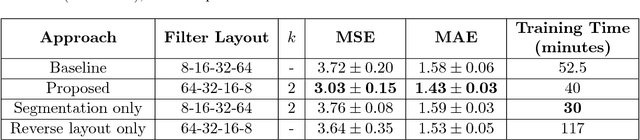
Abstract:Given the wide success of convolutional neural networks (CNNs) applied to natural images, researchers have begun to apply them to neuroimaging data. To date, however, exploration of novel CNN architectures tailored to neuroimaging data has been limited. Several recent works fail to leverage the 3D structure of the brain, instead treating the brain as a set of independent 2D slices. Approaches that do utilize 3D convolutions rely on architectures developed for object recognition tasks in natural 2D images. Such architectures make assumptions about the input that may not hold for neuroimaging. For example, existing architectures assume that patterns in the brain exhibit translation invariance. However, a pattern in the brain may have different meaning depending on where in the brain it is located. There is a need to explore novel architectures that are tailored to brain images. We present two simple modifications to existing CNN architectures based on brain image structure. Applied to the task of brain age prediction, our network achieves a mean absolute error (MAE) of 1.4 years and trains 30% faster than a CNN baseline that achieves a MAE of 1.6 years. Our results suggest that lessons learned from developing models on natural images may not directly transfer to neuroimaging tasks. Instead, there remains a large space of unexplored questions regarding model development in this area, whose answers may differ from conventional wisdom.
Disease Prediction based on Functional Connectomes using a Scalable and Spatially-Informed Support Vector Machine
Mar 25, 2014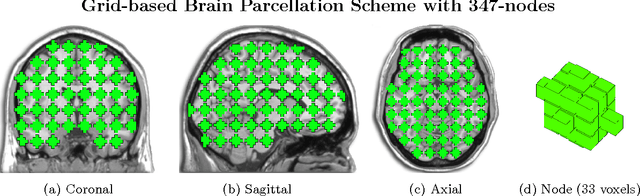
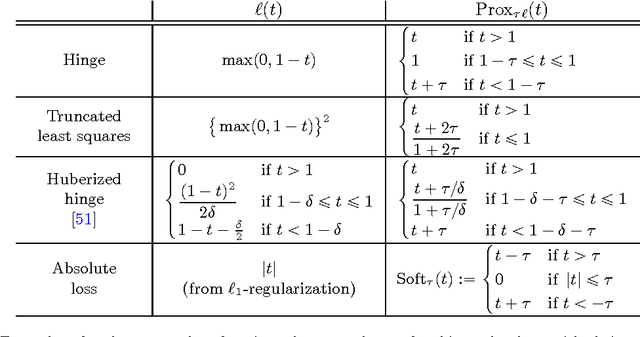
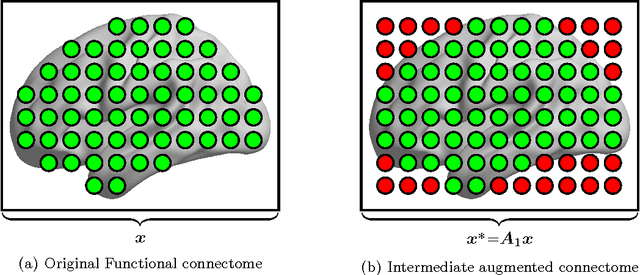

Abstract:Substantial evidence indicates that major psychiatric disorders are associated with distributed neural dysconnectivity, leading to strong interest in using neuroimaging methods to accurately predict disorder status. In this work, we are specifically interested in a multivariate approach that uses features derived from whole-brain resting state functional connectomes. However, functional connectomes reside in a high dimensional space, which complicates model interpretation and introduces numerous statistical and computational challenges. Traditional feature selection techniques are used to reduce data dimensionality, but are blind to the spatial structure of the connectomes. We propose a regularization framework where the 6-D structure of the functional connectome is explicitly taken into account via the fused Lasso or the GraphNet regularizer. Our method only restricts the loss function to be convex and margin-based, allowing non-differentiable loss functions such as the hinge-loss to be used. Using the fused Lasso or GraphNet regularizer with the hinge-loss leads to a structured sparse support vector machine (SVM) with embedded feature selection. We introduce a novel efficient optimization algorithm based on the augmented Lagrangian and the classical alternating direction method, which can solve both fused Lasso and GraphNet regularized SVM with very little modification. We also demonstrate that the inner subproblems of the algorithm can be solved efficiently in analytic form by coupling the variable splitting strategy with a data augmentation scheme. Experiments on simulated data and resting state scans from a large schizophrenia dataset show that our proposed approach can identify predictive regions that are spatially contiguous in the 6-D "connectome space," offering an additional layer of interpretability that could provide new insights about various disease processes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge