Ayhan Can Erdur
All Sizes Matter: Improving Volumetric Brain Segmentation on Small Lesions
Oct 04, 2023



Abstract:Brain metastases (BMs) are the most frequently occurring brain tumors. The treatment of patients having multiple BMs with stereo tactic radiosurgery necessitates accurate localization of the metastases. Neural networks can assist in this time-consuming and costly task that is typically performed by human experts. Particularly challenging is the detection of small lesions since they are often underrepresented in exist ing approaches. Yet, lesion detection is equally important for all sizes. In this work, we develop an ensemble of neural networks explicitly fo cused on detecting and segmenting small BMs. To accomplish this task, we trained several neural networks focusing on individual aspects of the BM segmentation problem: We use blob loss that specifically addresses the imbalance of lesion instances in terms of size and texture and is, therefore, not biased towards larger lesions. In addition, a model using a subtraction sequence between the T1 and T1 contrast-enhanced sequence focuses on low-contrast lesions. Furthermore, we train additional models only on small lesions. Our experiments demonstrate the utility of the ad ditional blob loss and the subtraction sequence. However, including the specialized small lesion models in the ensemble deteriorates segmentation results. We also find domain-knowledge-inspired postprocessing steps to drastically increase our performance in most experiments. Our approach enables us to submit a competitive challenge entry to the ASNR-MICCAI BraTS Brain Metastasis Challenge 2023.
Explainable 2D Vision Models for 3D Medical Data
Jul 13, 2023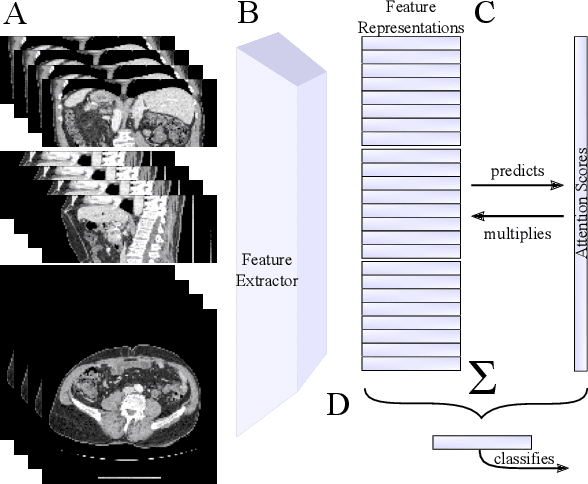
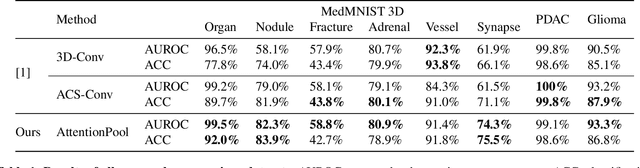
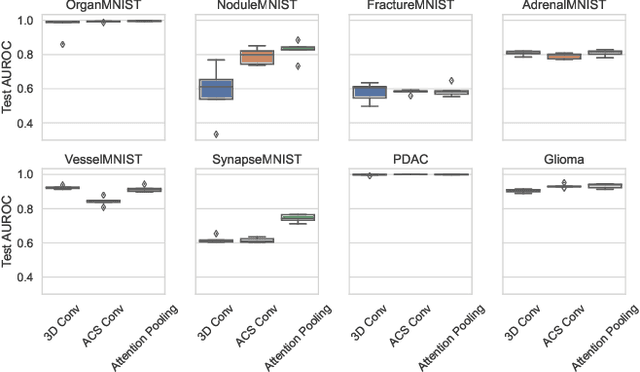
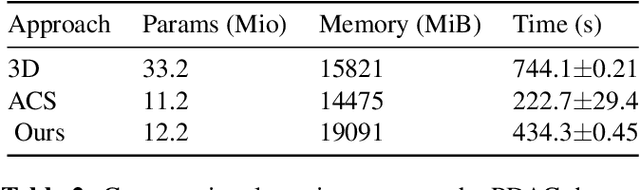
Abstract:Training Artificial Intelligence (AI) models on three-dimensional image data presents unique challenges compared to the two-dimensional case: Firstly, the computational resources are significantly higher, and secondly, the availability of large pretraining datasets is often limited, impeding training success. In this study, we propose a simple approach of adapting 2D networks with an intermediate feature representation for processing 3D volumes. Our method involves sequentially applying these networks to slices of a 3D volume from all orientations. Subsequently, a feature reduction module combines the extracted slice features into a single representation, which is then used for classification. We evaluate our approach on medical classification benchmarks and a real-world clinical dataset, demonstrating comparable results to existing methods. Furthermore, by employing attention pooling as a feature reduction module we obtain weighted importance values for each slice during the forward pass. We show that slices deemed important by our approach allow the inspection of the basis of a model's prediction.
Exploiting segmentation labels and representation learning to forecast therapy response of PDAC patients
Nov 08, 2022


Abstract:The prediction of pancreatic ductal adenocarcinoma therapy response is a clinically challenging and important task in this high-mortality tumour entity. The training of neural networks able to tackle this challenge is impeded by a lack of large datasets and the difficult anatomical localisation of the pancreas. Here, we propose a hybrid deep neural network pipeline to predict tumour response to initial chemotherapy which is based on the Response Evaluation Criteria in Solid Tumors (RECIST) score, a standardised method for cancer response evaluation by clinicians as well as tumour markers, and clinical evaluation of the patients. We leverage a combination of representation transfer from segmentation to classification, as well as localisation and representation learning. Our approach yields a remarkably data-efficient method able to predict treatment response with a ROC-AUC of 63.7% using only 477 datasets in total.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge