Archit Khosla
Improved statistical benchmarking of digital pathology models using pairwise frames evaluation
Jun 07, 2023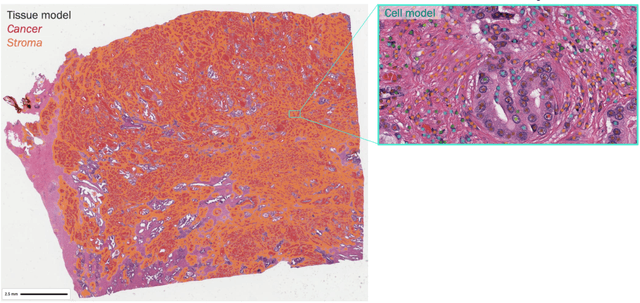
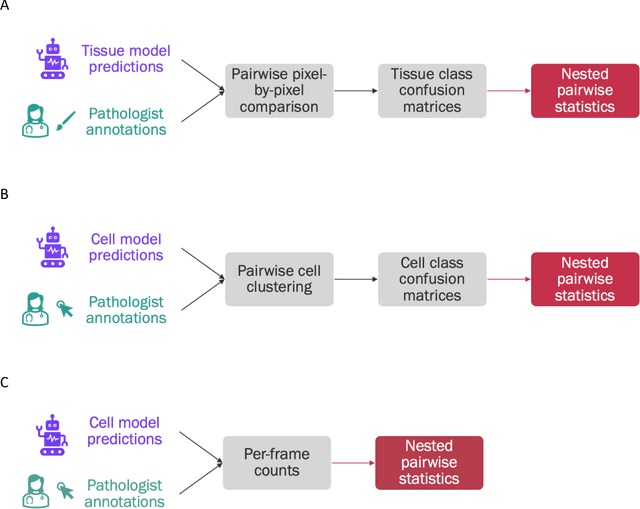
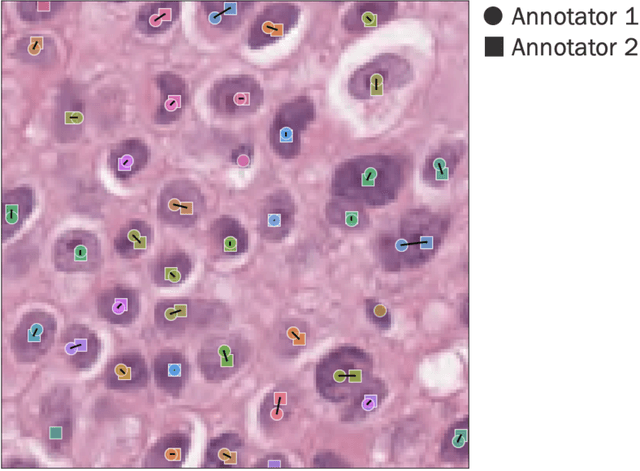
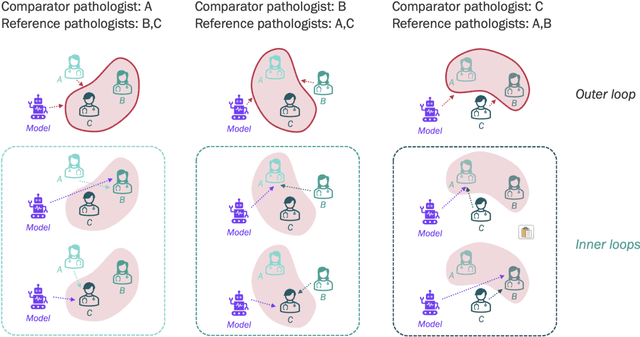
Abstract:Nested pairwise frames is a method for relative benchmarking of cell or tissue digital pathology models against manual pathologist annotations on a set of sampled patches. At a high level, the method compares agreement between a candidate model and pathologist annotations with agreement among pathologists' annotations. This evaluation framework addresses fundamental issues of data size and annotator variability in using manual pathologist annotations as a source of ground truth for model validation. We implemented nested pairwise frames evaluation for tissue classification, cell classification, and cell count prediction tasks and show results for cell and tissue models deployed on an H&E-stained melanoma dataset.
SC-MIL: Supervised Contrastive Multiple Instance Learning for Imbalanced Classification in Pathology
Mar 23, 2023Abstract:Multiple Instance learning (MIL) models have been extensively used in pathology to predict biomarkers and risk-stratify patients from gigapixel-sized images. Machine learning problems in medical imaging often deal with rare diseases, making it important for these models to work in a label-imbalanced setting. Furthermore, these imbalances can occur in out-of-distribution (OOD) datasets when the models are deployed in the real-world. We leverage the idea that decoupling feature and classifier learning can lead to improved decision boundaries for label imbalanced datasets. To this end, we investigate the integration of supervised contrastive learning with multiple instance learning (SC-MIL). Specifically, we propose a joint-training MIL framework in the presence of label imbalance that progressively transitions from learning bag-level representations to optimal classifier learning. We perform experiments with different imbalance settings for two well-studied problems in cancer pathology: subtyping of non-small cell lung cancer and subtyping of renal cell carcinoma. SC-MIL provides large and consistent improvements over other techniques on both in-distribution (ID) and OOD held-out sets across multiple imbalanced settings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge