Antonio Luiz P. Ribeiro
Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, Telehealth Center from Hospital das Clinicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
Detection of Chagas Disease from the ECG: The George B. Moody PhysioNet Challenge 2025
Oct 02, 2025Abstract:Objective: Chagas disease is a parasitic infection that is endemic to South America, Central America, and, more recently, the U.S., primarily transmitted by insects. Chronic Chagas disease can cause cardiovascular diseases and digestive problems. Serological testing capacities for Chagas disease are limited, but Chagas cardiomyopathy often manifests in ECGs, providing an opportunity to prioritize patients for testing and treatment. Approach: The George B. Moody PhysioNet Challenge 2025 invites teams to develop algorithmic approaches for identifying Chagas disease from electrocardiograms (ECGs). Main results: This Challenge provides multiple innovations. First, we leveraged several datasets with labels from patient reports and serological testing, provided a large dataset with weak labels and smaller datasets with strong labels. Second, we augmented the data to support model robustness and generalizability to unseen data sources. Third, we applied an evaluation metric that captured the local serological testing capacity for Chagas disease to frame the machine learning problem as a triage task. Significance: Over 630 participants from 111 teams submitted over 1300 entries during the Challenge, representing diverse approaches from academia and industry worldwide.
End-to-end Risk Prediction of Atrial Fibrillation from the 12-Lead ECG by Deep Neural Networks
Sep 28, 2023



Abstract:Background: Atrial fibrillation (AF) is one of the most common cardiac arrhythmias that affects millions of people each year worldwide and it is closely linked to increased risk of cardiovascular diseases such as stroke and heart failure. Machine learning methods have shown promising results in evaluating the risk of developing atrial fibrillation from the electrocardiogram. We aim to develop and evaluate one such algorithm on a large CODE dataset collected in Brazil. Results: The deep neural network model identified patients without indication of AF in the presented ECG but who will develop AF in the future with an AUC score of 0.845. From our survival model, we obtain that patients in the high-risk group (i.e. with the probability of a future AF case being greater than 0.7) are 50% more likely to develop AF within 40 weeks, while patients belonging to the minimal-risk group (i.e. with the probability of a future AF case being less than or equal to 0.1) have more than 85% chance of remaining AF free up until after seven years. Conclusion: We developed and validated a model for AF risk prediction. If applied in clinical practice, the model possesses the potential of providing valuable and useful information in decision-making and patient management processes.
* 16 pages with 7 figures
On Merging Feature Engineering and Deep Learning for Diagnosis, Risk-Prediction and Age Estimation Based on the 12-Lead ECG
Jul 16, 2022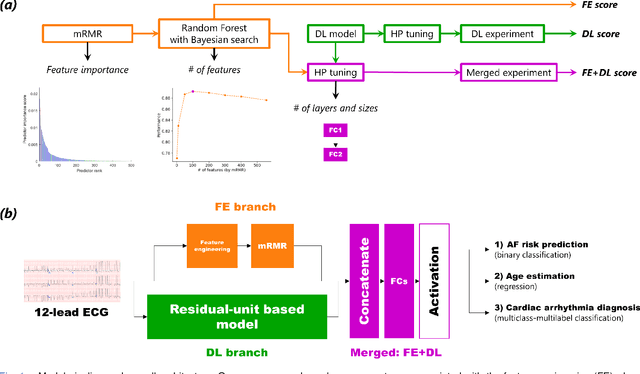
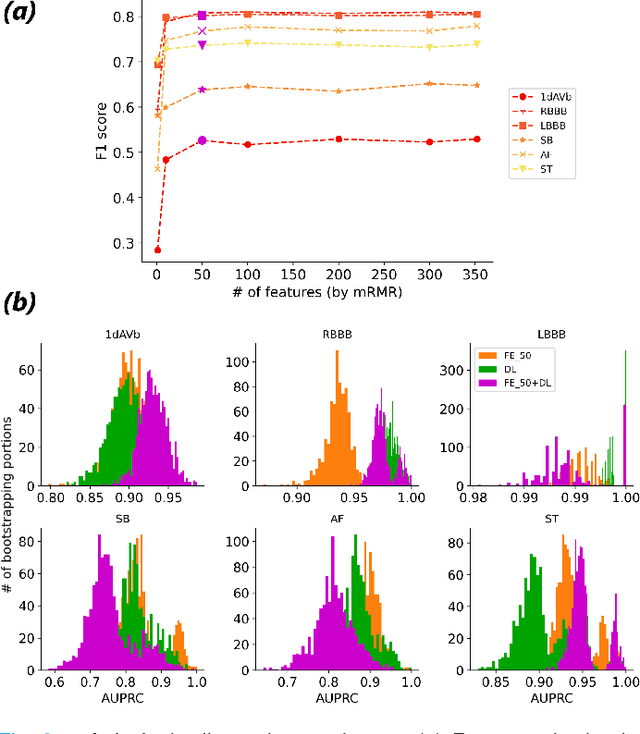
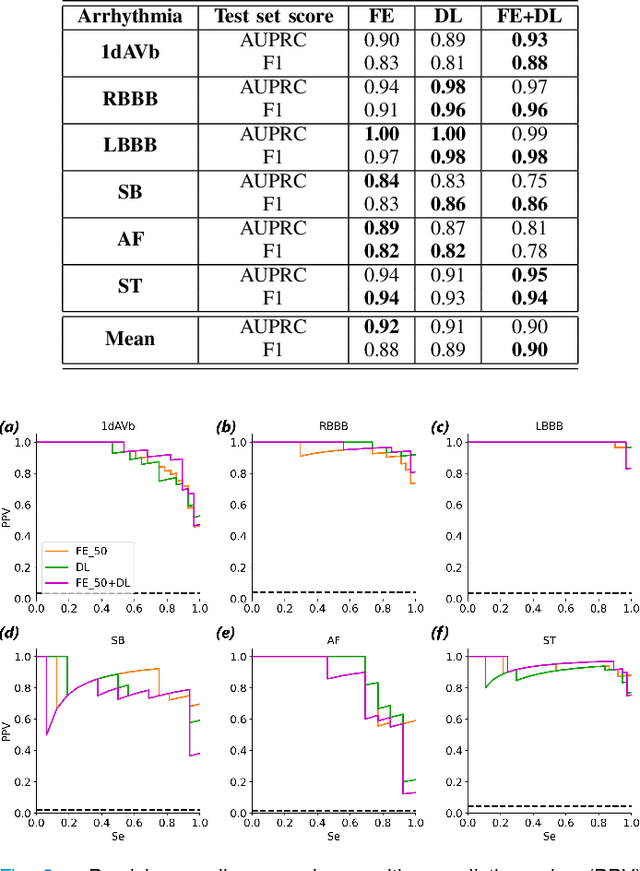
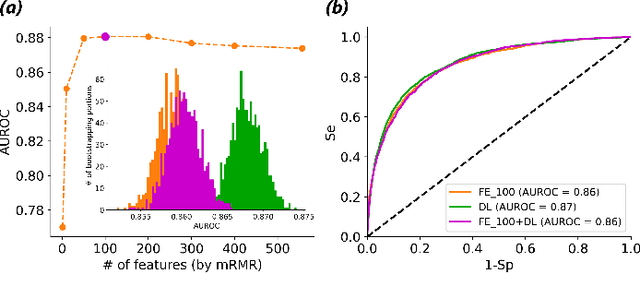
Abstract:Objective: Machine learning techniques have been used extensively for 12-lead electrocardiogram (ECG) analysis. For physiological time series, deep learning (DL) superiority to feature engineering (FE) approaches based on domain knowledge is still an open question. Moreover, it remains unclear whether combining DL with FE may improve performance. Methods: We considered three tasks intending to address these research gaps: cardiac arrhythmia diagnosis (multiclass-multilabel classification), atrial fibrillation risk prediction (binary classification), and age estimation (regression). We used an overall dataset of 2.3M 12-lead ECG recordings to train the following models for each task: i) a random forest taking the FE as input was trained as a classical machine learning approach; ii) an end-to-end DL model; and iii) a merged model of FE+DL. Results: FE yielded comparable results to DL while necessitating significantly less data for the two classification tasks and it was outperformed by DL for the regression task. For all tasks, merging FE with DL did not improve performance over DL alone. Conclusion: We found that for traditional 12-lead ECG based diagnosis tasks DL did not yield a meaningful improvement over FE, while it improved significantly the nontraditional regression task. We also found that combining FE with DL did not improve over DL alone which suggests that the FE were redundant with the features learned by DL. Significance: Our findings provides important recommendations on what machine learning strategy and data regime to chose with respect to the task at hand for the development of new machine learning models based on the 12-lead ECG.
Automatic Diagnosis of the Short-Duration 12-Lead ECG using a Deep Neural Network: the CODE Study
Apr 02, 2019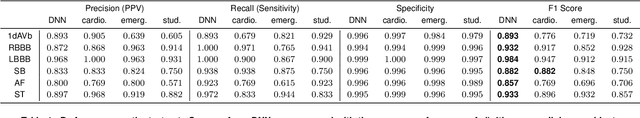
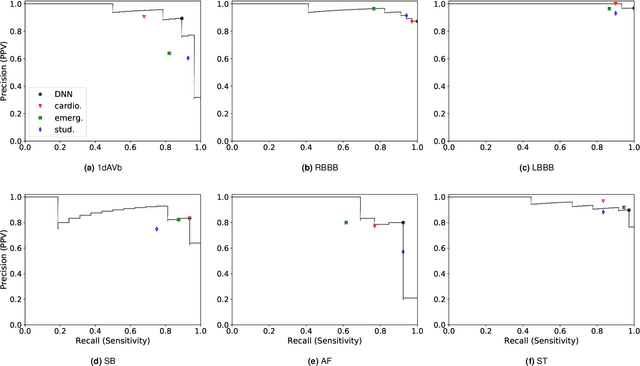
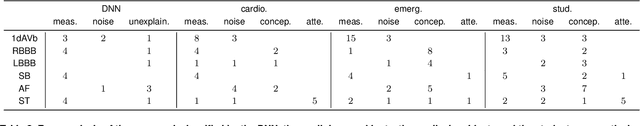
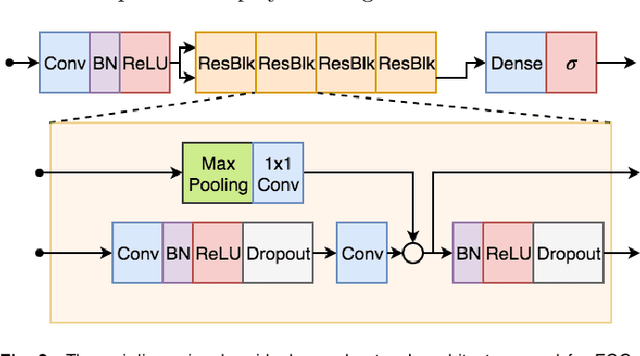
Abstract:We present a Deep Neural Network (DNN) model for predicting electrocardiogram (ECG) abnormalities in short-duration 12-lead ECG recordings. The analysis of the digital ECG obtained in a clinical setting can provide a full evaluation of the cardiac electrical activity and have not been studied in an end-to-end machine learning scenario. Using the database of the Telehealth Network of Minas Gerais, under the scope of the CODE (Clinical Outcomes in Digital Electrocardiology) study, we built a novel dataset with more than 2 million ECG tracings, orders of magnitude larger than those used in previous studies. Moreover, our dataset is more realistic, as it consists of 12-lead ECGs recorded during standard in-clinic exams. Using this data, we trained a residual neural network with 9 convolutional layers to map ECG signals with a duration of 7 to 10 seconds into 6 different classes of ECG abnormalities. High-performance measures were obtained for all ECG abnormalities, with F1 scores above $80\%$ and specificity indexes over $99\%$. We compare the performance with cardiology and emergency resident medical doctors as well as medical students and, considering the F1 score, the DNN matches or outperforms the medical residents and students for all abnormalities. These results indicate that end-to-end automatic ECG analysis based on DNNs, previously used only in a single-lead setup, generalizes well to the 12-lead ECG. This is an important result in that it takes this technology much closer to standard clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge