Alexey Shevtsov
The impact of deep learning aid on the workload and interpretation accuracy of radiologists on chest computed tomography: a cross-over reader study
Jun 12, 2024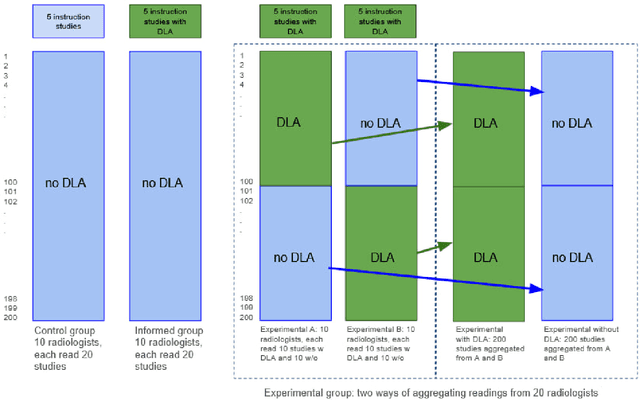
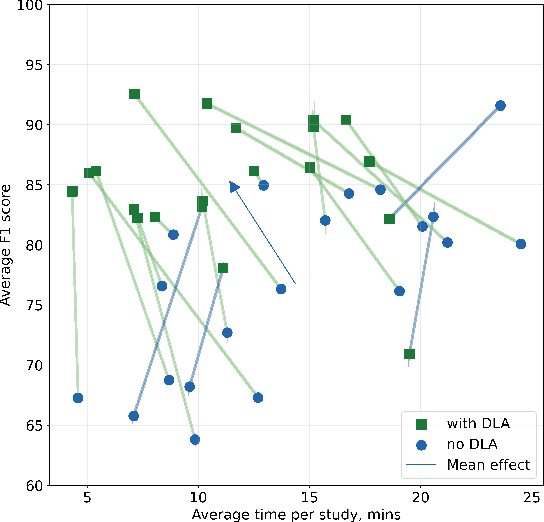
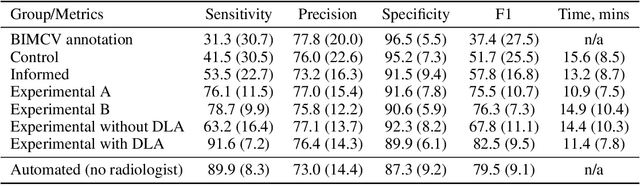
Abstract:Interpretation of chest computed tomography (CT) is time-consuming. Previous studies have measured the time-saving effect of using a deep-learning-based aid (DLA) for CT interpretation. We evaluated the joint impact of a multi-pathology DLA on the time and accuracy of radiologists' reading. 40 radiologists were randomly split into three experimental arms: control (10), who interpret studies without assistance; informed group (10), who were briefed about DLA pathologies, but performed readings without it; and the experimental group (20), who interpreted half studies with DLA, and half without. Every arm used the same 200 CT studies retrospectively collected from BIMCV-COVID19 dataset; each radiologist provided readings for 20 CT studies. We compared interpretation time, and accuracy of participants diagnostic report with respect to 12 pathological findings. Mean reading time per study was 15.6 minutes [SD 8.5] in the control arm, 13.2 minutes [SD 8.7] in the informed arm, 14.4 [SD 10.3] in the experimental arm without DLA, and 11.4 minutes [SD 7.8] in the experimental arm with DLA. Mean sensitivity and specificity were 41.5 [SD 30.4], 86.8 [SD 28.3] in the control arm; 53.5 [SD 22.7], 92.3 [SD 9.4] in the informed non-assisted arm; 63.2 [SD 16.4], 92.3 [SD 8.2] in the experimental arm without DLA; and 91.6 [SD 7.2], 89.9 [SD 6.0] in the experimental arm with DLA. DLA speed up interpretation time per study by 2.9 minutes (CI95 [1.7, 4.3], p<0.0005), increased sensitivity by 28.4 (CI95 [23.4, 33.4], p<0.0005), and decreased specificity by 2.4 (CI95 [0.6, 4.3], p=0.13). Of 20 radiologists in the experimental arm, 16 have improved reading time and sensitivity, two improved their time with a marginal drop in sensitivity, and two participants improved sensitivity with increased time. Overall, DLA introduction decreased reading time by 20.6%.
Systematic Clinical Evaluation of A Deep Learning Method for Medical Image Segmentation: Radiosurgery Application
Aug 21, 2021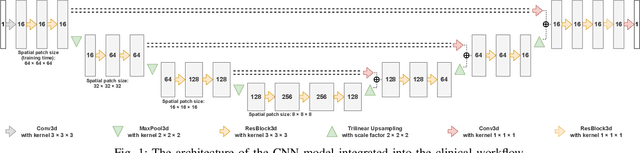
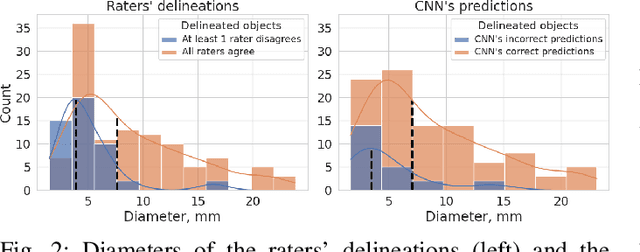
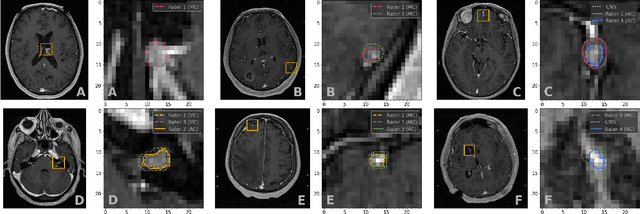
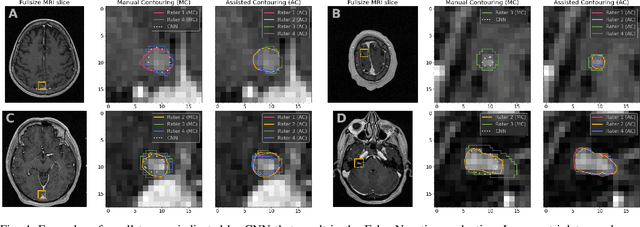
Abstract:We systematically evaluate a Deep Learning (DL) method in a 3D medical image segmentation task. Our segmentation method is integrated into the radiosurgery treatment process and directly impacts the clinical workflow. With our method, we address the relative drawbacks of manual segmentation: high inter-rater contouring variability and high time consumption of the contouring process. The main extension over the existing evaluations is the careful and detailed analysis that could be further generalized on other medical image segmentation tasks. Firstly, we analyze the changes in the inter-rater detection agreement. We show that the segmentation model reduces the ratio of detection disagreements from 0.162 to 0.085 (p < 0.05). Secondly, we show that the model improves the inter-rater contouring agreement from 0.845 to 0.871 surface Dice Score (p < 0.05). Thirdly, we show that the model accelerates the delineation process in between 1.6 and 2.0 times (p < 0.05). Finally, we design the setup of the clinical experiment to either exclude or estimate the evaluation biases, thus preserve the significance of the results. Besides the clinical evaluation, we also summarize the intuitions and practical ideas for building an efficient DL-based model for 3D medical image segmentation.
Universal Loss Reweighting to Balance Lesion Size Inequality in 3D Medical Image Segmentation
Jul 20, 2020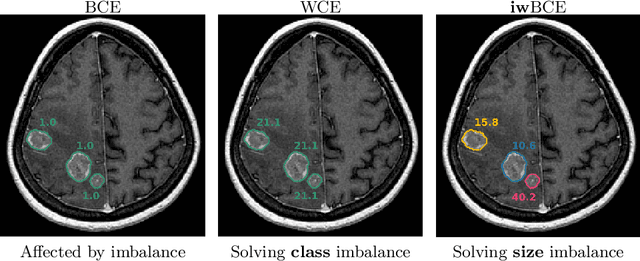
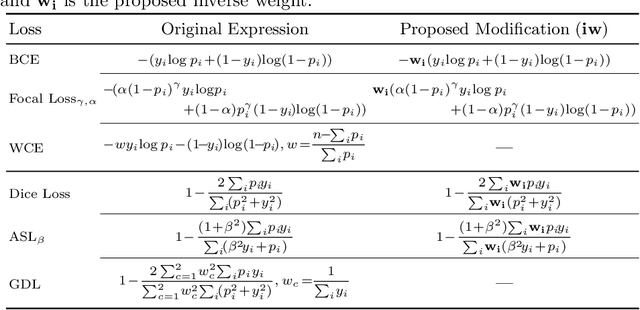
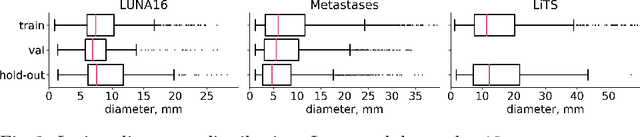
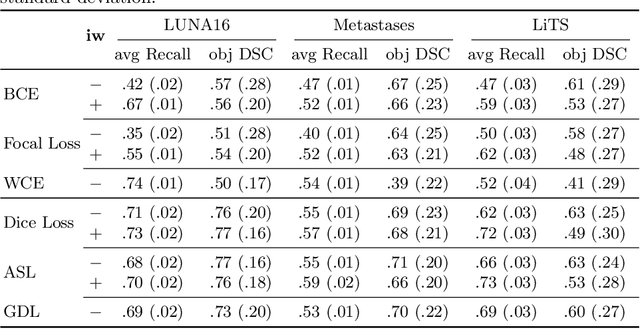
Abstract:Target imbalance affects the performance of recent deep learning methods in many medical image segmentation tasks. It is a twofold problem: class imbalance - positive class (lesion) size compared to negative class (non-lesion) size; lesion size imbalance - large lesions overshadows small ones (in the case of multiple lesions per image). While the former was addressed in multiple works, the latter lacks investigation. We propose a loss reweighting approach to increase the ability of the network to detect small lesions. During the learning process, we assign a weight to every image voxel. The assigned weights are inversely proportional to the lesion volume, thus smaller lesions get larger weights. We report the benefit from our method for well-known loss functions, including Dice Loss, Focal Loss, and Asymmetric Similarity Loss. Additionally, we compare our results with other reweighting techniques: Weighted Cross-Entropy and Generalized Dice Loss. Our experiments show that inverse weighting considerably increases the detection quality, while preserves the delineation quality on a state-of-the-art level. We publish a complete experimental pipeline for two publicly available datasets of CT images: LiTS and LUNA16 (https://github.com/neuro-ml/inverse_weighting). We also show results on a private database of MR images for the task of multiple brain metastases delineation.
CT-based COVID-19 Triage: Deep Multitask Learning Improves Joint Identification and Severity Quantification
Jun 02, 2020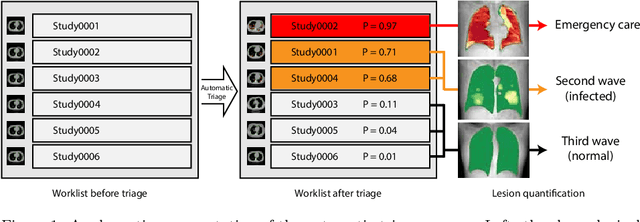

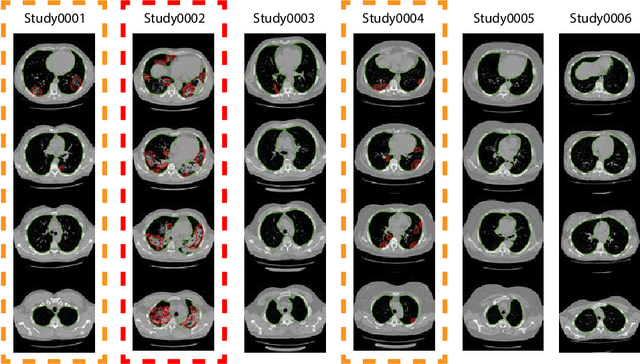

Abstract:The current COVID-19 pandemic overloads healthcare systems, including radiology departments. Though several deep learning approaches were developed to assist in CT analysis, nobody considered study triage directly as a computer science problem. We describe two basic setups: Identification of COVID-19 to prioritize studies of potentially infected patients to isolate them as early as possible; Severity quantification to highlight studies of severe patients and direct them to a hospital or provide emergency medical care. We formalize these tasks as binary classification and estimation of affected lung percentage. Though similar problems were well-studied separately, we show that existing methods provide reasonable quality only for one of these setups. To consolidate both triage approaches, we employ a multitask learning and propose a convolutional neural network to combine all available labels within a single model. We train our model on approximately 2000 publicly available CT studies and test it with a carefully designed set consisting of 33 COVID patients, 32 healthy patients, and 36 patients with other lung pathologies to emulate a typical patient flow in an out-patient hospital. The developed model achieved 0.951 ROC AUC for Identification of COVID-19 and 0.98 Spearman Correlation for Severity quantification. We release all the code and create a public leaderboard, where other community members can test their models on our dataset.
Deep Learning for Brain Tumor Segmentation in Radiosurgery: Prospective Clinical Evaluation
Sep 06, 2019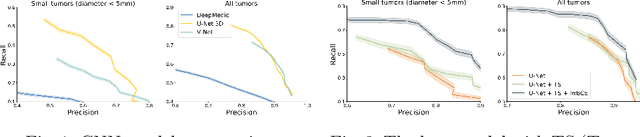
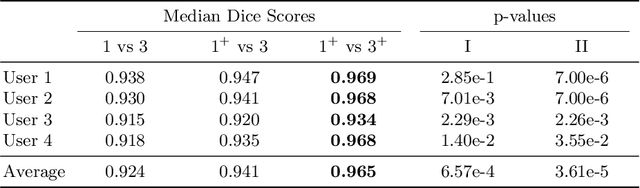
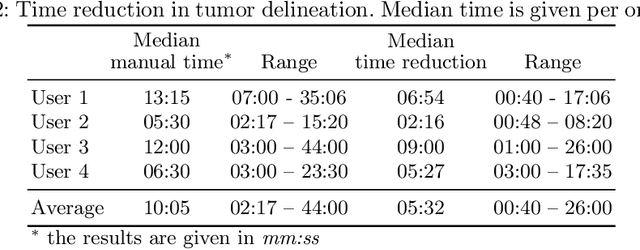
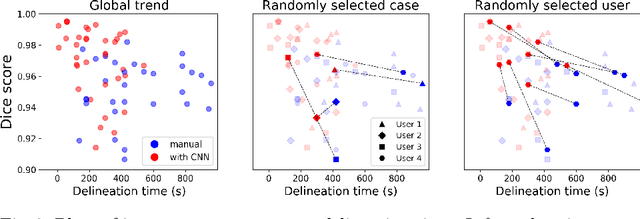
Abstract:Stereotactic radiosurgery is a minimally-invasive treatment option for a large number of patients with intracranial tumors. As part of the therapy treatment, accurate delineation of brain tumors is of great importance. However, slice-by-slice manual segmentation on T1c MRI could be time-consuming (especially for multiple metastases) and subjective (especially for meningiomas). In our work, we compared several deep convolutional networks architectures and training procedures and evaluated the best model in a radiation therapy department for three types of brain tumors: meningiomas, schwannomas and multiple brain metastases. The developed semiautomatic segmentation system accelerates the contouring process by 2.2 times on average and increases inter-rater agreement from 92% to 96.5%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge