Sergey Morozov
Interpretable Vertebral Fracture Quantification via Anchor-Free Landmarks Localization
Apr 14, 2022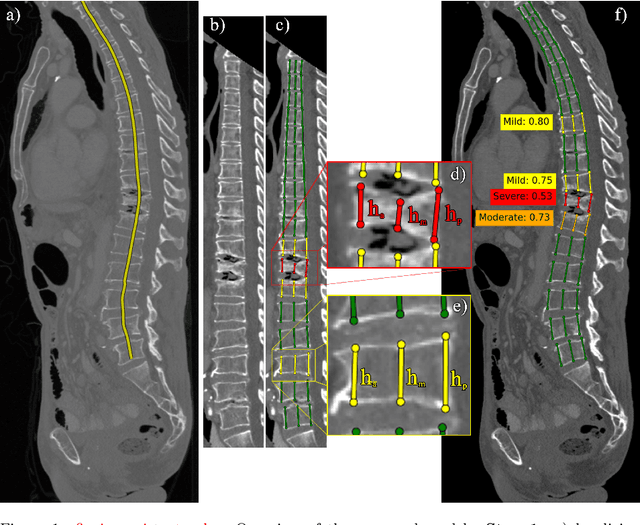
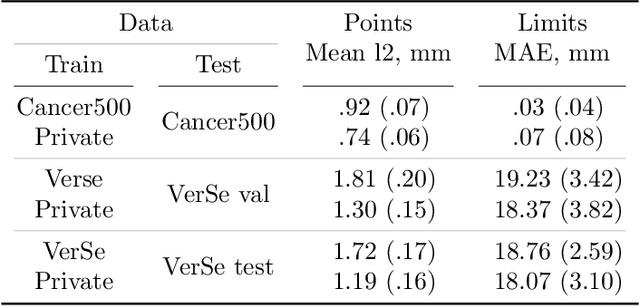
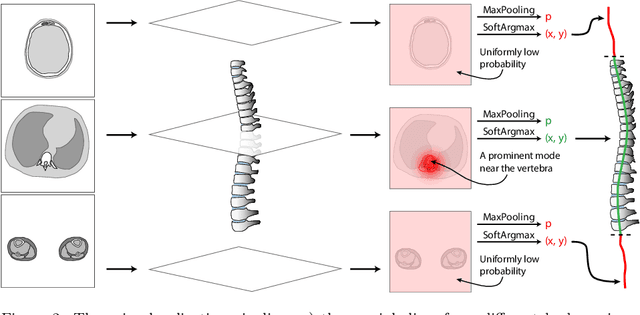
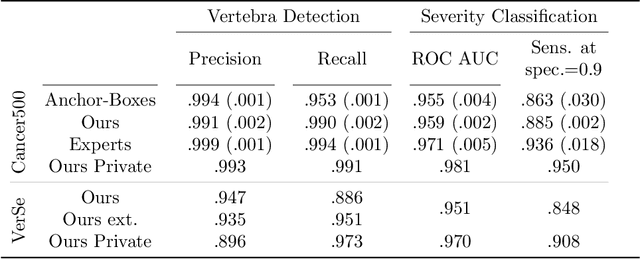
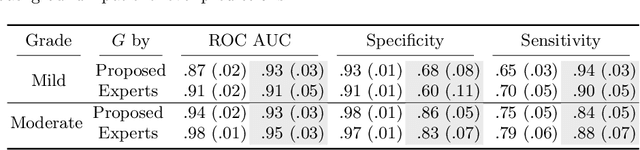
Abstract:Vertebral body compression fractures are early signs of osteoporosis. Though these fractures are visible on Computed Tomography (CT) images, they are frequently missed by radiologists in clinical settings. Prior research on automatic methods of vertebral fracture classification proves its reliable quality; however, existing methods provide hard-to-interpret outputs and sometimes fail to process cases with severe abnormalities such as highly pathological vertebrae or scoliosis. We propose a new two-step algorithm to localize the vertebral column in 3D CT images and then detect individual vertebrae and quantify fractures in 2D simultaneously. We train neural networks for both steps using a simple 6-keypoints based annotation scheme, which corresponds precisely to the current clinical recommendation. Our algorithm has no exclusion criteria, processes 3D CT in 2 seconds on a single GPU, and provides an interpretable and verifiable output. The method approaches expert-level performance and demonstrates state-of-the-art results in vertebrae 3D localization (the average error is 1 mm), vertebrae 2D detection (precision and recall are 0.99), and fracture identification (ROC AUC at the patient level is up to 0.96). Our anchor-free vertebra detection network shows excellent generalizability on a new domain by achieving ROC AUC 0.95, sensitivity 0.85, specificity 0.9 on a challenging VerSe dataset with many unseen vertebra types.
CT-based COVID-19 Triage: Deep Multitask Learning Improves Joint Identification and Severity Quantification
Jun 02, 2020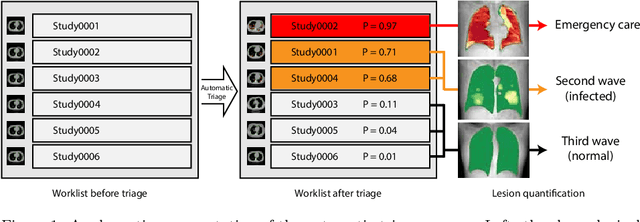

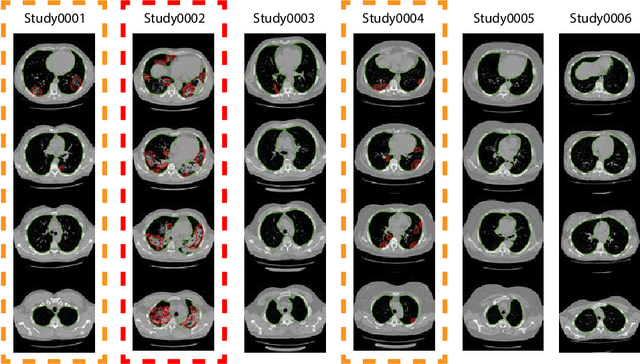

Abstract:The current COVID-19 pandemic overloads healthcare systems, including radiology departments. Though several deep learning approaches were developed to assist in CT analysis, nobody considered study triage directly as a computer science problem. We describe two basic setups: Identification of COVID-19 to prioritize studies of potentially infected patients to isolate them as early as possible; Severity quantification to highlight studies of severe patients and direct them to a hospital or provide emergency medical care. We formalize these tasks as binary classification and estimation of affected lung percentage. Though similar problems were well-studied separately, we show that existing methods provide reasonable quality only for one of these setups. To consolidate both triage approaches, we employ a multitask learning and propose a convolutional neural network to combine all available labels within a single model. We train our model on approximately 2000 publicly available CT studies and test it with a carefully designed set consisting of 33 COVID patients, 32 healthy patients, and 36 patients with other lung pathologies to emulate a typical patient flow in an out-patient hospital. The developed model achieved 0.951 ROC AUC for Identification of COVID-19 and 0.98 Spearman Correlation for Severity quantification. We release all the code and create a public leaderboard, where other community members can test their models on our dataset.
Keypoints Localization for Joint Vertebra Detection and Fracture Severity Quantification
May 25, 2020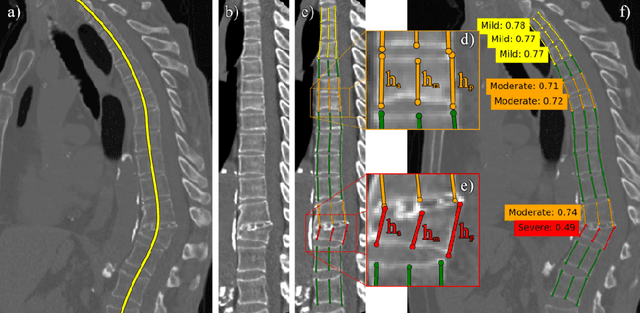
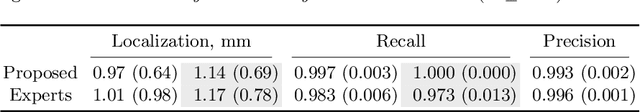
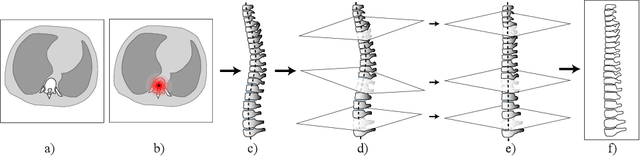
Abstract:Vertebral body compression fractures are reliable early signs of osteoporosis. Though these fractures are visible on Computed Tomography (CT) images, they are frequently missed by radiologists in clinical settings. Prior research on automatic methods of vertebral fracture classification proves its reliable quality; however, existing methods provide hard-to-interpret outputs and sometimes fail to process cases with severe abnormalities such as highly pathological vertebrae or scoliosis. We propose a new two-step algorithm to localize the vertebral column in 3D CT images and then to simultaneously detect individual vertebrae and quantify fractures in 2D. We train neural networks for both steps using a simple 6-keypoints based annotation scheme, which corresponds precisely to current medical recommendation. Our algorithm has no exclusion criteria, processes 3D CT in 2 seconds on a single GPU, and provides an intuitive and verifiable output. The method approaches expert-level performance and demonstrates state-of-the-art results in vertebrae 3D localization (the average error is 1 mm), vertebrae 2D detection (precision is 0.99, recall is 1), and fracture identification (ROC AUC at the patient level is 0.93).
Incorporating Task-Specific Structural Knowledge into CNNs for Brain Midline Shift Detection
Aug 13, 2019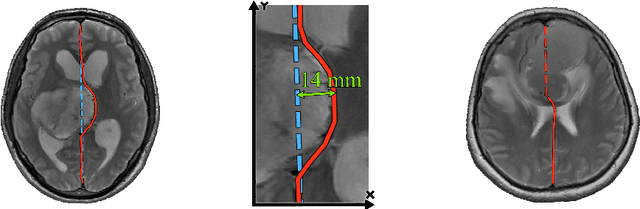

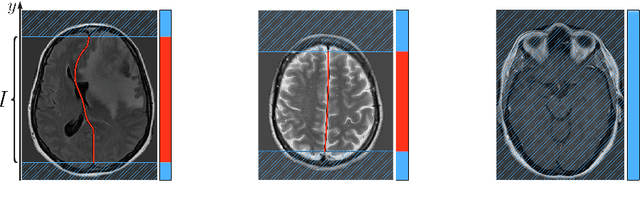
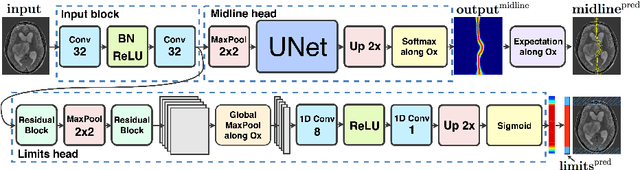
Abstract:Midline shift (MLS) is a well-established factor used for outcome prediction in traumatic brain injury, stroke and brain tumors. The importance of automatic estimation of MLS was recently highlighted by ACR Data Science Institute. In this paper we introduce a novel deep learning based approach for the problem of MLS detection, which exploits task-specific structural knowledge. We evaluate our method on a large dataset containing heterogeneous images with significant MLS and show that its mean error approaches the inter-expert variability. Finally, we show the robustness of our approach by validating it on an external dataset, acquired during routine clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge