Ziad Al-Haj Hemidi
PrIINeR: Towards Prior-Informed Implicit Neural Representations for Accelerated MRI
Aug 11, 2025Abstract:Accelerating Magnetic Resonance Imaging (MRI) reduces scan time but often degrades image quality. While Implicit Neural Representations (INRs) show promise for MRI reconstruction, they struggle at high acceleration factors due to weak prior constraints, leading to structural loss and aliasing artefacts. To address this, we propose PrIINeR, an INR-based MRI reconstruction method that integrates prior knowledge from pre-trained deep learning models into the INR framework. By combining population-level knowledge with instance-based optimization and enforcing dual data consistency, PrIINeR aligns both with the acquired k-space data and the prior-informed reconstruction. Evaluated on the NYU fastMRI dataset, our method not only outperforms state-of-the-art INR-based approaches but also improves upon several learning-based state-of-the-art methods, significantly improving structural preservation and fidelity while effectively removing aliasing artefacts.PrIINeR bridges deep learning and INR-based techniques, offering a more reliable solution for high-quality, accelerated MRI reconstruction. The code is publicly available on https://github.com/multimodallearning/PrIINeR.
IM-MoCo: Self-supervised MRI Motion Correction using Motion-Guided Implicit Neural Representations
Jul 03, 2024Abstract:Motion artifacts in Magnetic Resonance Imaging (MRI) arise due to relatively long acquisition times and can compromise the clinical utility of acquired images. Traditional motion correction methods often fail to address severe motion, leading to distorted and unreliable results. Deep Learning (DL) alleviated such pitfalls through generalization with the cost of vanishing structures and hallucinations, making it challenging to apply in the medical field where hallucinated structures can tremendously impact the diagnostic outcome. In this work, we present an instance-wise motion correction pipeline that leverages motion-guided Implicit Neural Representations (INRs) to mitigate the impact of motion artifacts while retaining anatomical structure. Our method is evaluated using the NYU fastMRI dataset with different degrees of simulated motion severity. For the correction alone, we can improve over state-of-the-art image reconstruction methods by $+5\%$ SSIM, $+5\:db$ PSNR, and $+14\%$ HaarPSI. Clinical relevance is demonstrated by a subsequent experiment, where our method improves classification outcomes by at least $+1.5$ accuracy percentage points compared to motion-corrupted images.
The state-of-the-art in Cardiac MRI Reconstruction: Results of the CMRxRecon Challenge in MICCAI 2023
Apr 01, 2024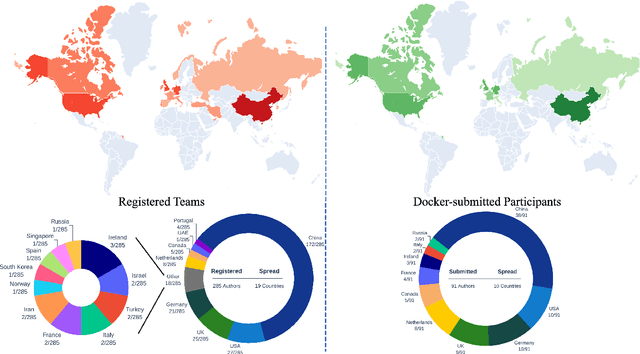
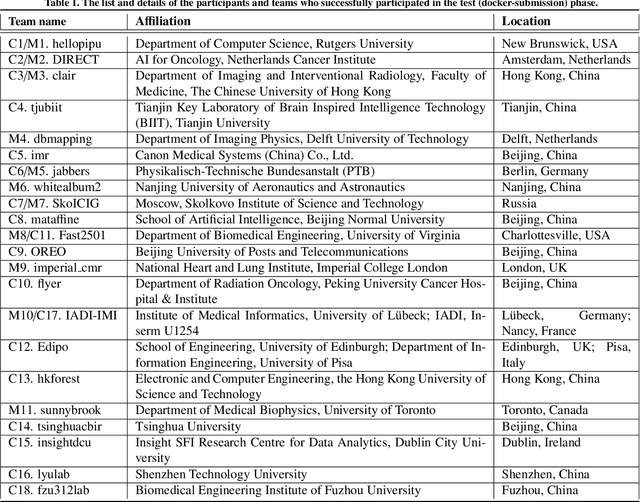
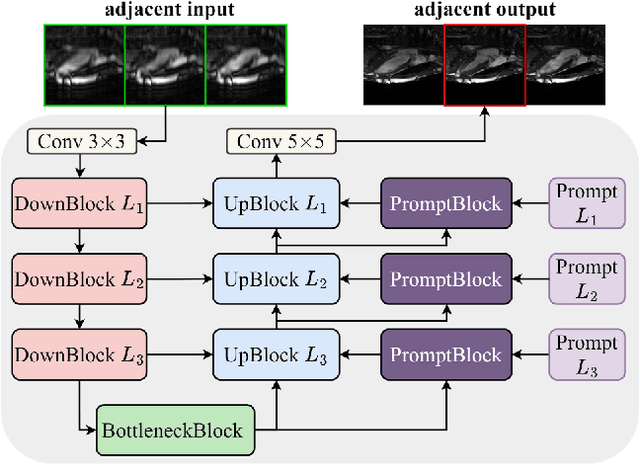
Abstract:Cardiac MRI, crucial for evaluating heart structure and function, faces limitations like slow imaging and motion artifacts. Undersampling reconstruction, especially data-driven algorithms, has emerged as a promising solution to accelerate scans and enhance imaging performance using highly under-sampled data. Nevertheless, the scarcity of publicly available cardiac k-space datasets and evaluation platform hinder the development of data-driven reconstruction algorithms. To address this issue, we organized the Cardiac MRI Reconstruction Challenge (CMRxRecon) in 2023, in collaboration with the 26th International Conference on MICCAI. CMRxRecon presented an extensive k-space dataset comprising cine and mapping raw data, accompanied by detailed annotations of cardiac anatomical structures. With overwhelming participation, the challenge attracted more than 285 teams and over 600 participants. Among them, 22 teams successfully submitted Docker containers for the testing phase, with 7 teams submitted for both cine and mapping tasks. All teams use deep learning based approaches, indicating that deep learning has predominately become a promising solution for the problem. The first-place winner of both tasks utilizes the E2E-VarNet architecture as backbones. In contrast, U-Net is still the most popular backbone for both multi-coil and single-coil reconstructions. This paper provides a comprehensive overview of the challenge design, presents a summary of the submitted results, reviews the employed methods, and offers an in-depth discussion that aims to inspire future advancements in cardiac MRI reconstruction models. The summary emphasizes the effective strategies observed in Cardiac MRI reconstruction, including backbone architecture, loss function, pre-processing techniques, physical modeling, and model complexity, thereby providing valuable insights for further developments in this field.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge