Yuchi Han
Motion-Guided Deep Image Prior for Cardiac MRI
Dec 05, 2024



Abstract:Cardiovascular magnetic resonance imaging is a powerful diagnostic tool for assessing cardiac structure and function. Traditional breath-held imaging protocols, however, pose challenges for patients with arrhythmias or limited breath-holding capacity. We introduce Motion-Guided Deep Image prior (M-DIP), a novel unsupervised reconstruction framework for accelerated real-time cardiac MRI. M-DIP employs a spatial dictionary to synthesize a time-dependent template image, which is further refined using time-dependent deformation fields that model cardiac and respiratory motion. Unlike prior DIP-based methods, M-DIP simultaneously captures physiological motion and frame-to-frame content variations, making it applicable to a wide range of dynamic applications. We validate M-DIP using simulated MRXCAT cine phantom data as well as free-breathing real-time cine and single-shot late gadolinium enhancement data from clinical patients. Comparative analyses against state-of-the-art supervised and unsupervised approaches demonstrate M-DIP's performance and versatility. M-DIP achieved better image quality metrics on phantom data, as well as higher reader scores for in-vivo patient data.
Lost in Tracking: Uncertainty-guided Cardiac Cine MRI Segmentation at Right Ventricle Base
Oct 04, 2024Abstract:Accurate biventricular segmentation of cardiac magnetic resonance (CMR) cine images is essential for the clinical evaluation of heart function. However, compared to left ventricle (LV), right ventricle (RV) segmentation is still more challenging and less reproducible. Degenerate performance frequently occurs at the RV base, where the in-plane anatomical structures are complex (with atria, valve, and aorta) and vary due to the strong interplanar motion. In this work, we propose to address the currently unsolved issues in CMR segmentation, specifically at the RV base, with two strategies: first, we complemented the public resource by reannotating the RV base in the ACDC dataset, with refined delineation of the right ventricle outflow tract (RVOT), under the guidance of an expert cardiologist. Second, we proposed a novel dual encoder U-Net architecture that leverages temporal incoherence to inform the segmentation when interplanar motions occur. The inter-planar motion is characterized by loss-of-tracking, via Bayesian uncertainty of a motion-tracking model. Our experiments showed that our method significantly improved RV base segmentation taking into account temporal incoherence. Furthermore, we investigated the reproducibility of deep learning-based segmentation and showed that the combination of consistent annotation and loss of tracking could enhance the reproducibility of RV segmentation, potentially facilitating a large number of clinical studies focusing on RV.
Coil Reweighting to Suppress Motion Artifacts in Real-Time Exercise Cine Imaging
May 26, 2024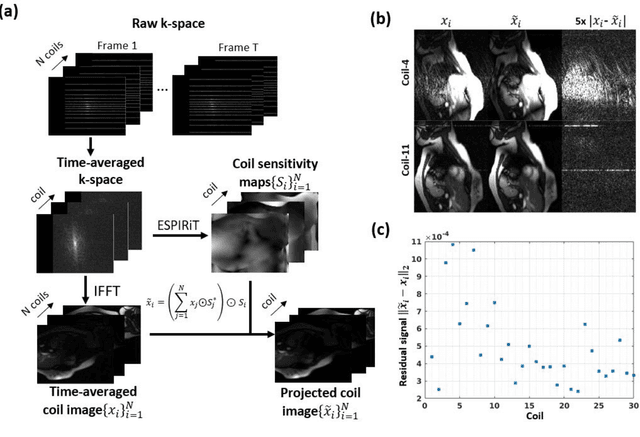
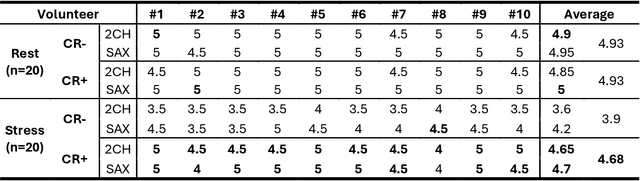
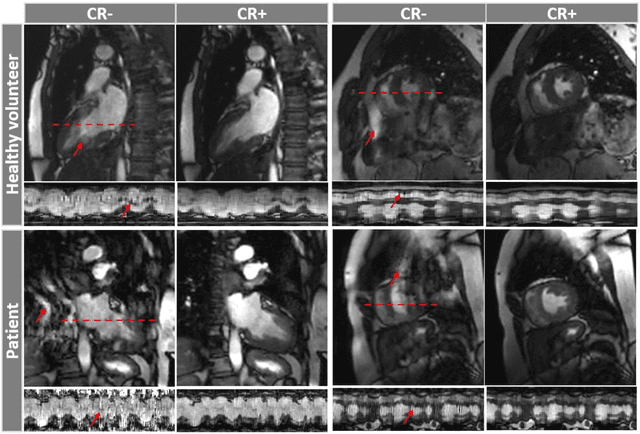
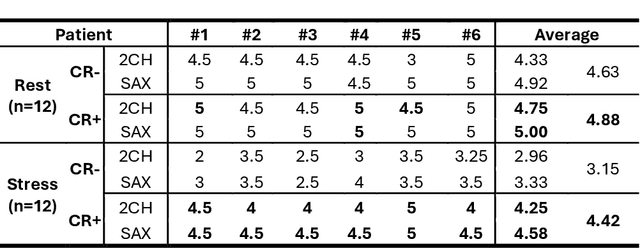
Abstract:Background: Accelerated real-time cine (RT-Cine) imaging enables cardiac function assessment without the need for breath-holding. However, when performed during in-magnet exercise, RT-Cine images may exhibit significant motion artifacts. Methods: By projecting the time-averaged images to the subspace spanned by the coil sensitivity maps, we propose a coil reweighting (CR) method to automatically suppress a subset of receive coils that introduces a high level of artifacts in the reconstructed image. RT-Cine data collected at rest and during exercise from ten healthy volunteers and six patients were utilized to assess the performance of the proposed method. One short-axis and one two-chamber RT-Cine series reconstructed with and without CR from each subject were visually scored by two cardiologists in terms of the level of artifacts on a scale of 1 (worst) to 5 (best). Results: For healthy volunteers, applying CR to RT-Cine images collected at rest did not significantly change the image quality score (p=1). In contrast, for RT-Cine images collected during exercise, CR significantly improved the score from 3.9 to 4.68 (p<0.001). Similarly, in patients, CR did not significantly change the score for images collected at rest (p=0.031) but markedly improved the score from 3.15 to 4.42 (p<0.001) for images taken during exercise. Despite lower image quality scores in the patient cohort compared to healthy subjects, likely due to larger body habitus and the difficulty of limiting body motion during exercise, CR effectively suppressed motion artifacts, with all image series from the patient cohort receiving a score of four or higher. Conclusion: Using data from healthy subjects and patients, we demonstrate that the motion artifacts in the reconstructed RT-Cine images can be effectively suppressed significantly with the proposed CR method.
Accelerated Real-time Cine and Flow under In-magnet Staged Exercise
Feb 27, 2024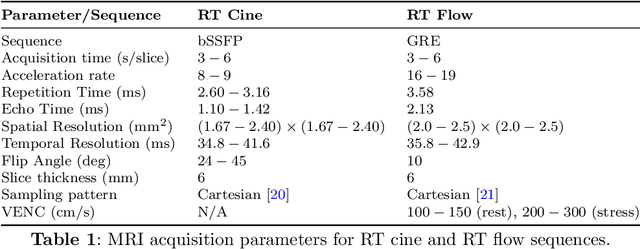
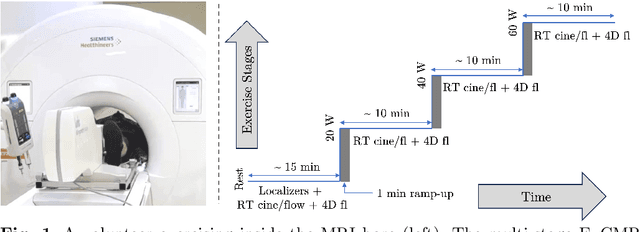
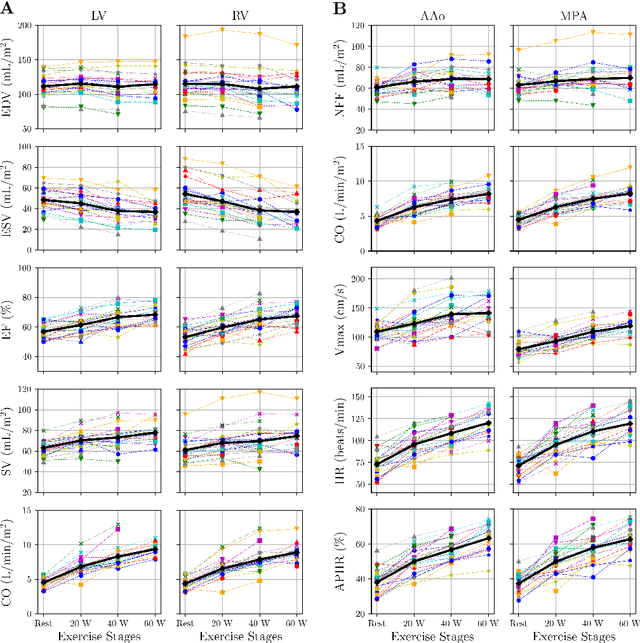
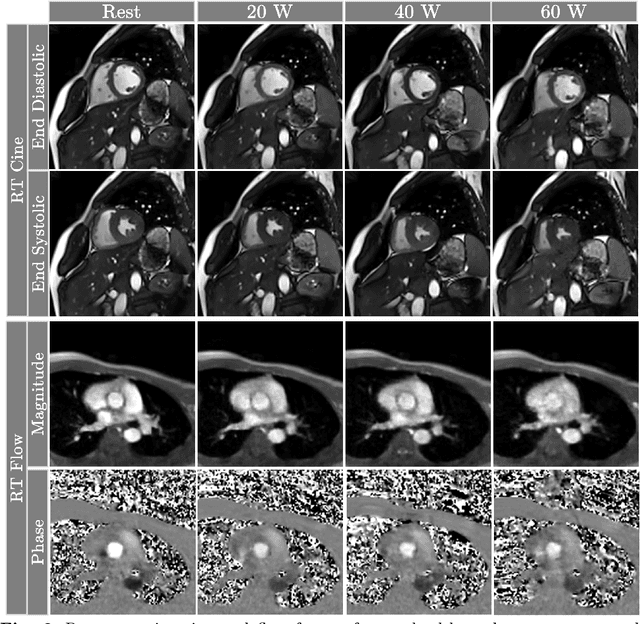
Abstract:Background: Cardiovascular magnetic resonance imaging (CMR) is a well-established imaging tool for diagnosing and managing cardiac conditions. The integration of exercise stress with CMR (ExCMR) can enhance its diagnostic capacity. Despite recent advances in CMR technology, ExCMR remains technically challenging due to motion artifacts and limited spatial and temporal resolution. Methods: This study investigates the feasibility of biventricular functional and hemodynamic assessment using real-time (RT) ExCMR during a staged exercise protocol in 26 healthy volunteers. We introduce a coil reweighting technique to minimize motion artifacts. In addition, we identify and analyze heartbeats from the end-expiratory phase to enhance the repeatability of cardiac function quantification. To demonstrate clinical feasibility, qualitative results from five patients are also presented. Results: Our findings indicate a consistent decrease in end-systolic volume (ESV) and stable end-diastolic volume (EDV) across exercise intensities, leading to increased stroke volume (SV) and ejection fraction (EF). Coil reweighting effectively reduces motion artifacts, improving image quality in both healthy volunteers and patients. The repeatability of cardiac function parameters, demonstrated by scan-rescan tests in nine volunteers, improves with the selection of end-expiratory beats. Conclusions: The study demonstrates that RT ExCMR with in-magnet exercise is a feasible and effective method for dynamic cardiac function monitoring during exercise. The proposed coil reweighting technique and selection of end-expiratory beats significantly enhance image quality and repeatability.
Motion-robust free-running cardiovascular MRI
Aug 04, 2023Abstract:PURPOSE: To present and validate an outlier rejection method that makes free-running cardiovascular MRI (CMR) more motion robust. METHODS: The proposed method, called compressive recovery with outlier rejection (CORe), models outliers as an auxiliary variable that is added to the measured data. We enforce MR physics-guided group-sparsity on the auxiliary variable and jointly estimate it along with the image using an iterative algorithm. For validation, CORe is first compared to traditional compressed sensing (CS), robust regression (RR), and another outlier rejection method using two simulation studies. Then, CORe is compared to CS using five 3D cine and ten rest and stress 4D flow imaging datasets. RESULTS: Our simulation studies show that CORe outperforms CS, RR, and the outlier rejection method in terms of normalized mean squared error (NMSE) and structural similarity index (SSIM) across 50 different realizations. The expert reader evaluation of 3D cine images demonstrates that CORe is more effective in suppressing artifacts while maintaining or improving image sharpness. The flow consistency evaluation in 4D flow images show that CORe yields more consistent flow measurements, especially under exercise stress. CONCLUSION: An outlier rejection method is presented and validated using simulated and measured data. This method can help suppress motion artifacts in a wide range of free-running CMR applications. CODE: MATLAB implementation code is available on GitHub at https://github.com/syedmurtazaarshad/motion-robust-CMR
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge