Syed Murtaza Arshad
Motion-Guided Deep Image Prior for Cardiac MRI
Dec 05, 2024



Abstract:Cardiovascular magnetic resonance imaging is a powerful diagnostic tool for assessing cardiac structure and function. Traditional breath-held imaging protocols, however, pose challenges for patients with arrhythmias or limited breath-holding capacity. We introduce Motion-Guided Deep Image prior (M-DIP), a novel unsupervised reconstruction framework for accelerated real-time cardiac MRI. M-DIP employs a spatial dictionary to synthesize a time-dependent template image, which is further refined using time-dependent deformation fields that model cardiac and respiratory motion. Unlike prior DIP-based methods, M-DIP simultaneously captures physiological motion and frame-to-frame content variations, making it applicable to a wide range of dynamic applications. We validate M-DIP using simulated MRXCAT cine phantom data as well as free-breathing real-time cine and single-shot late gadolinium enhancement data from clinical patients. Comparative analyses against state-of-the-art supervised and unsupervised approaches demonstrate M-DIP's performance and versatility. M-DIP achieved better image quality metrics on phantom data, as well as higher reader scores for in-vivo patient data.
Accelerated Real-time Cine and Flow under In-magnet Staged Exercise
Feb 27, 2024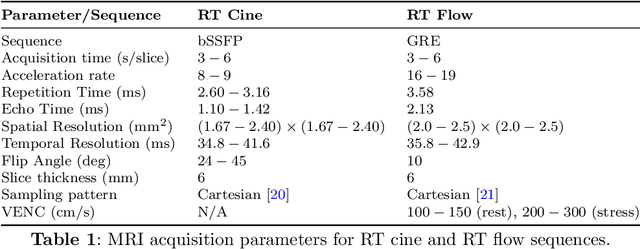
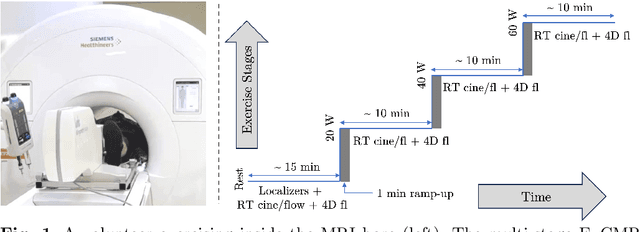
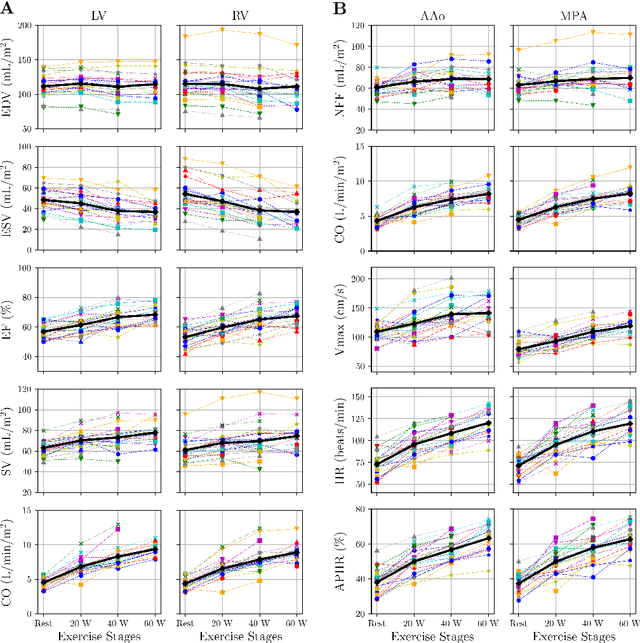
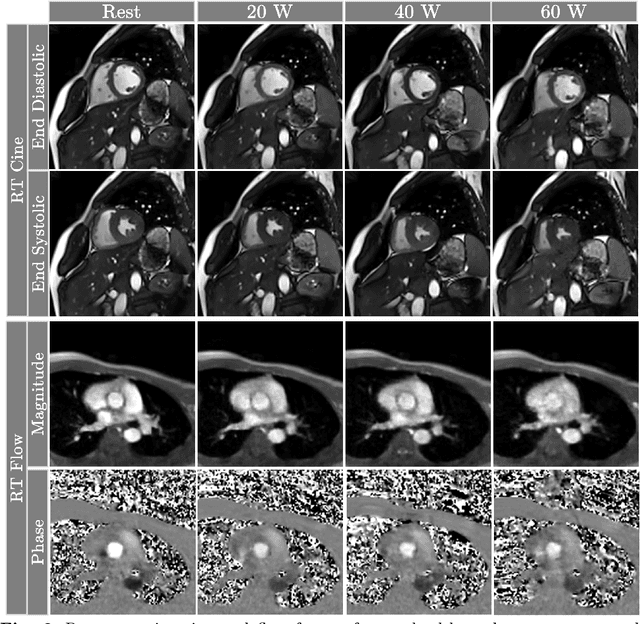
Abstract:Background: Cardiovascular magnetic resonance imaging (CMR) is a well-established imaging tool for diagnosing and managing cardiac conditions. The integration of exercise stress with CMR (ExCMR) can enhance its diagnostic capacity. Despite recent advances in CMR technology, ExCMR remains technically challenging due to motion artifacts and limited spatial and temporal resolution. Methods: This study investigates the feasibility of biventricular functional and hemodynamic assessment using real-time (RT) ExCMR during a staged exercise protocol in 26 healthy volunteers. We introduce a coil reweighting technique to minimize motion artifacts. In addition, we identify and analyze heartbeats from the end-expiratory phase to enhance the repeatability of cardiac function quantification. To demonstrate clinical feasibility, qualitative results from five patients are also presented. Results: Our findings indicate a consistent decrease in end-systolic volume (ESV) and stable end-diastolic volume (EDV) across exercise intensities, leading to increased stroke volume (SV) and ejection fraction (EF). Coil reweighting effectively reduces motion artifacts, improving image quality in both healthy volunteers and patients. The repeatability of cardiac function parameters, demonstrated by scan-rescan tests in nine volunteers, improves with the selection of end-expiratory beats. Conclusions: The study demonstrates that RT ExCMR with in-magnet exercise is a feasible and effective method for dynamic cardiac function monitoring during exercise. The proposed coil reweighting technique and selection of end-expiratory beats significantly enhance image quality and repeatability.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge