Yingmin Liu
An unsupervised method for MRI recovery: Deep image prior with structured sparsity
Jan 02, 2025



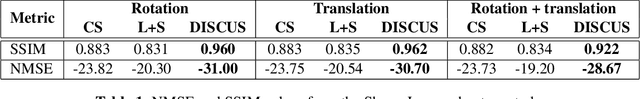
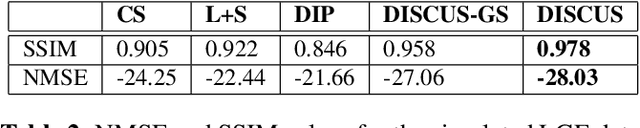
Abstract:Objective: To propose and validate an unsupervised MRI reconstruction method that does not require fully sampled k-space data. Materials and Methods: The proposed method, deep image prior with structured sparsity (DISCUS), extends the deep image prior (DIP) by introducing group sparsity to frame-specific code vectors, enabling the discovery of a low-dimensional manifold for capturing temporal variations. \discus was validated using four studies: (I) simulation of a dynamic Shepp-Logan phantom to demonstrate its manifold discovery capabilities, (II) comparison with compressed sensing and DIP-based methods using simulated single-shot late gadolinium enhancement (LGE) image series from six distinct digital cardiac phantoms in terms of normalized mean square error (NMSE) and structural similarity index measure (SSIM), (III) evaluation on retrospectively undersampled single-shot LGE data from eight patients, and (IV) evaluation on prospectively undersampled single-shot LGE data from eight patients, assessed via blind scoring from two expert readers. Results: DISCUS outperformed competing methods, demonstrating superior reconstruction quality in terms of NMSE and SSIM (Studies I--III) and expert reader scoring (Study IV). Discussion: An unsupervised image reconstruction method is presented and validated on simulated and measured data. These developments can benefit applications where acquiring fully sampled data is challenging.
Coil Reweighting to Suppress Motion Artifacts in Real-Time Exercise Cine Imaging
May 26, 2024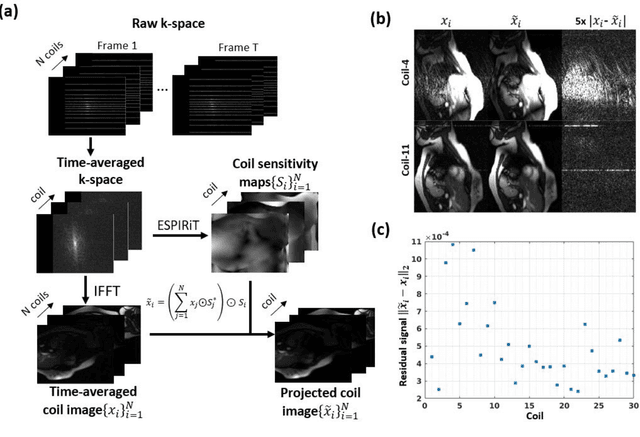
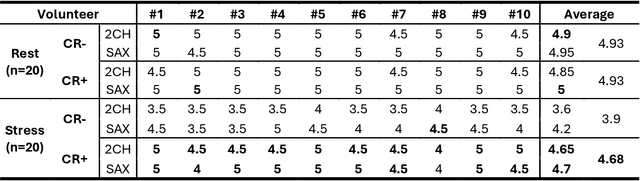
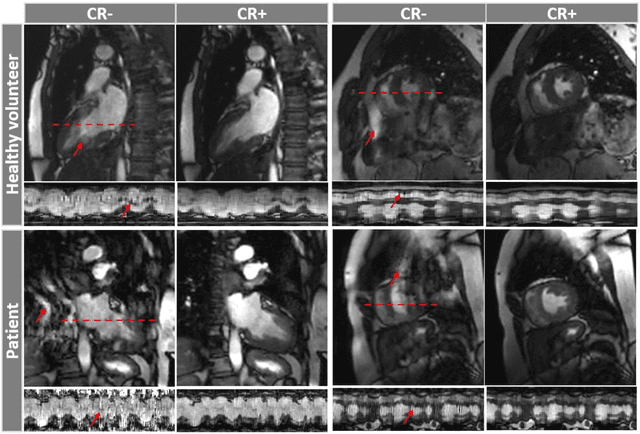
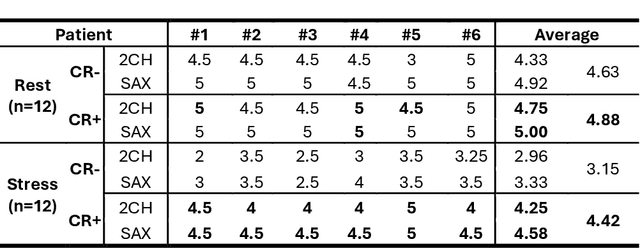
Abstract:Background: Accelerated real-time cine (RT-Cine) imaging enables cardiac function assessment without the need for breath-holding. However, when performed during in-magnet exercise, RT-Cine images may exhibit significant motion artifacts. Methods: By projecting the time-averaged images to the subspace spanned by the coil sensitivity maps, we propose a coil reweighting (CR) method to automatically suppress a subset of receive coils that introduces a high level of artifacts in the reconstructed image. RT-Cine data collected at rest and during exercise from ten healthy volunteers and six patients were utilized to assess the performance of the proposed method. One short-axis and one two-chamber RT-Cine series reconstructed with and without CR from each subject were visually scored by two cardiologists in terms of the level of artifacts on a scale of 1 (worst) to 5 (best). Results: For healthy volunteers, applying CR to RT-Cine images collected at rest did not significantly change the image quality score (p=1). In contrast, for RT-Cine images collected during exercise, CR significantly improved the score from 3.9 to 4.68 (p<0.001). Similarly, in patients, CR did not significantly change the score for images collected at rest (p=0.031) but markedly improved the score from 3.15 to 4.42 (p<0.001) for images taken during exercise. Despite lower image quality scores in the patient cohort compared to healthy subjects, likely due to larger body habitus and the difficulty of limiting body motion during exercise, CR effectively suppressed motion artifacts, with all image series from the patient cohort receiving a score of four or higher. Conclusion: Using data from healthy subjects and patients, we demonstrate that the motion artifacts in the reconstructed RT-Cine images can be effectively suppressed significantly with the proposed CR method.
Accelerated Real-time Cine and Flow under In-magnet Staged Exercise
Feb 27, 2024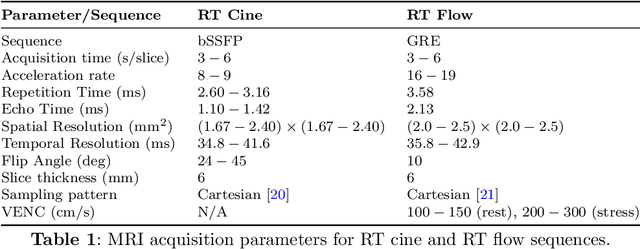
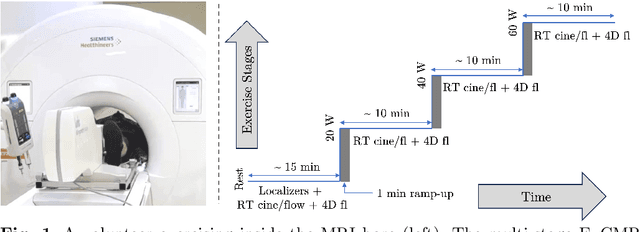
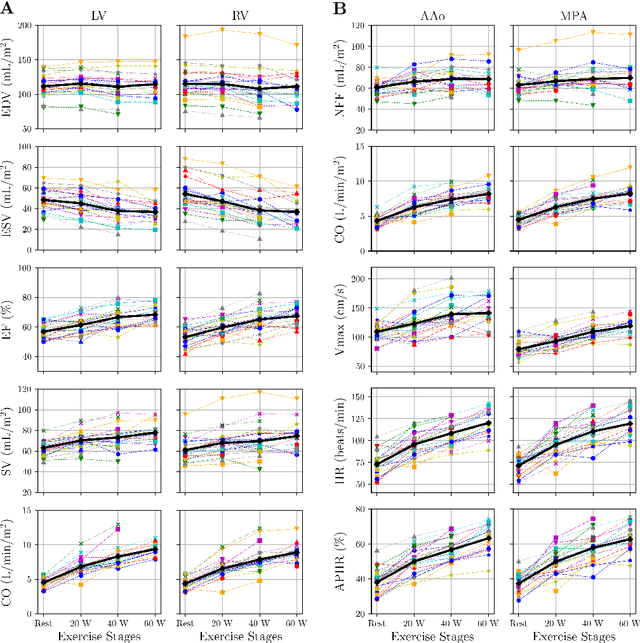
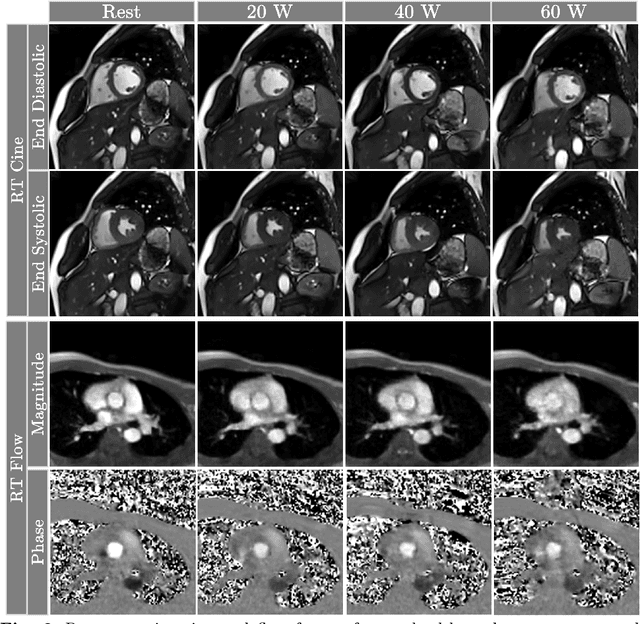
Abstract:Background: Cardiovascular magnetic resonance imaging (CMR) is a well-established imaging tool for diagnosing and managing cardiac conditions. The integration of exercise stress with CMR (ExCMR) can enhance its diagnostic capacity. Despite recent advances in CMR technology, ExCMR remains technically challenging due to motion artifacts and limited spatial and temporal resolution. Methods: This study investigates the feasibility of biventricular functional and hemodynamic assessment using real-time (RT) ExCMR during a staged exercise protocol in 26 healthy volunteers. We introduce a coil reweighting technique to minimize motion artifacts. In addition, we identify and analyze heartbeats from the end-expiratory phase to enhance the repeatability of cardiac function quantification. To demonstrate clinical feasibility, qualitative results from five patients are also presented. Results: Our findings indicate a consistent decrease in end-systolic volume (ESV) and stable end-diastolic volume (EDV) across exercise intensities, leading to increased stroke volume (SV) and ejection fraction (EF). Coil reweighting effectively reduces motion artifacts, improving image quality in both healthy volunteers and patients. The repeatability of cardiac function parameters, demonstrated by scan-rescan tests in nine volunteers, improves with the selection of end-expiratory beats. Conclusions: The study demonstrates that RT ExCMR with in-magnet exercise is a feasible and effective method for dynamic cardiac function monitoring during exercise. The proposed coil reweighting technique and selection of end-expiratory beats significantly enhance image quality and repeatability.
Deep Image prior with StruCtUred Sparsity for dynamic MRI reconstruction
Dec 01, 2023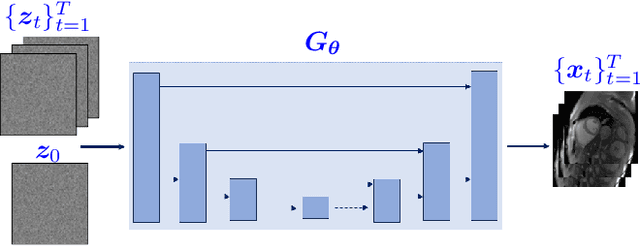
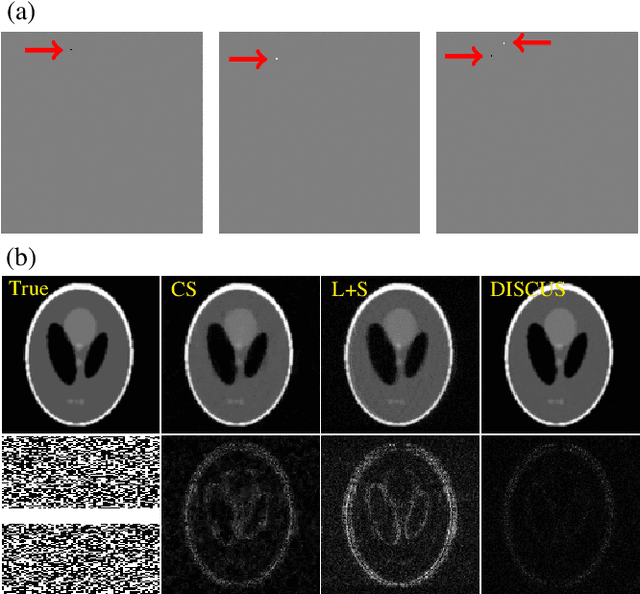
Abstract:High-quality training data are not always available in dynamic MRI. To address this, we propose a self-supervised deep learning method called deep image prior with structured sparsity (DISCUS) for reconstructing dynamic images. DISCUS is inspired by deep image prior (DIP) and recovers a series of images through joint optimization of network parameters and input code vectors. However, DISCUS additionally encourages group sparsity on frame-specific code vectors to discover the low-dimensional manifold that describes temporal variations across frames. Compared to prior work on manifold learning, DISCUS does not require specifying the manifold dimensionality. We validate DISCUS using three numerical studies. In the first study, we simulate a dynamic Shepp-Logan phantom with frames undergoing random rotations, translations, or both, and demonstrate that DISCUS can discover the dimensionality of the underlying manifold. In the second study, we use data from a realistic late gadolinium enhancement (LGE) phantom to compare DISCUS with compressed sensing (CS) and DIP and to demonstrate the positive impact of group sparsity. In the third study, we use retrospectively undersampled single-shot LGE data from five patients to compare DISCUS with CS reconstructions. The results from these studies demonstrate that DISCUS outperforms CS and DIP and that enforcing group sparsity on the code vectors helps discover true manifold dimensionality and provides additional performance gain.
Motion-robust free-running cardiovascular MRI
Aug 04, 2023Abstract:PURPOSE: To present and validate an outlier rejection method that makes free-running cardiovascular MRI (CMR) more motion robust. METHODS: The proposed method, called compressive recovery with outlier rejection (CORe), models outliers as an auxiliary variable that is added to the measured data. We enforce MR physics-guided group-sparsity on the auxiliary variable and jointly estimate it along with the image using an iterative algorithm. For validation, CORe is first compared to traditional compressed sensing (CS), robust regression (RR), and another outlier rejection method using two simulation studies. Then, CORe is compared to CS using five 3D cine and ten rest and stress 4D flow imaging datasets. RESULTS: Our simulation studies show that CORe outperforms CS, RR, and the outlier rejection method in terms of normalized mean squared error (NMSE) and structural similarity index (SSIM) across 50 different realizations. The expert reader evaluation of 3D cine images demonstrates that CORe is more effective in suppressing artifacts while maintaining or improving image sharpness. The flow consistency evaluation in 4D flow images show that CORe yields more consistent flow measurements, especially under exercise stress. CONCLUSION: An outlier rejection method is presented and validated using simulated and measured data. This method can help suppress motion artifacts in a wide range of free-running CMR applications. CODE: MATLAB implementation code is available on GitHub at https://github.com/syedmurtazaarshad/motion-robust-CMR
Technical Report (v1.0)--Pseudo-random Cartesian Sampling for Dynamic MRI
Jun 08, 2022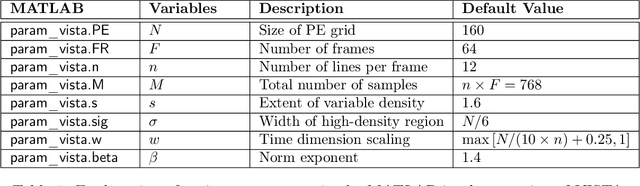
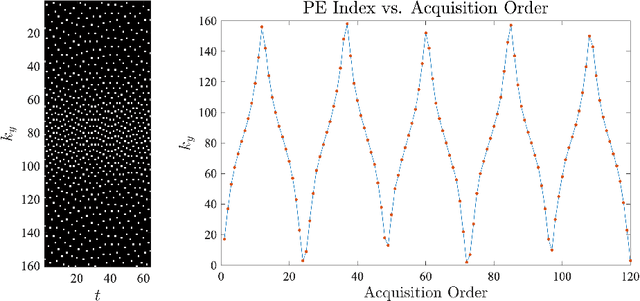
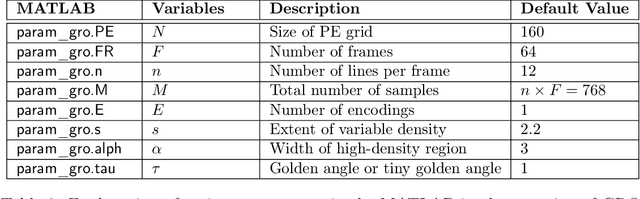
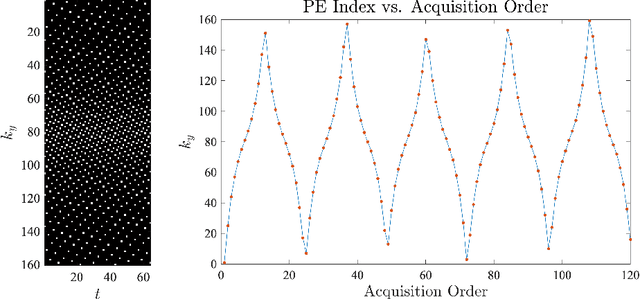
Abstract:For an effective application of compressed sensing (CS), which exploits the underlying compressibility of an image, one of the requirements is that the undersampling artifact be incoherent (noise-like) in the sparsifying transform domain. For cardiovascular MRI (CMR), several pseudo-random sampling methods have been proposed that yield a high level of incoherence. In this technical report, we present a collection of five pseudo-random Cartesian sampling methods that can be applied to 2D cine and flow, 3D volumetric cine, and 4D flow imaging. Four out of the five presented methods yield fast computation for on-the-fly generation of the sampling mask, without the need to create and store pre-computed look-up tables. In addition, the sampling distribution is parameterized, providing control over the sampling density. For each sampling method in the report, (i) we briefly describe the methodology, (ii) list default values of the pertinent parameters, and (iii) provide a publicly available MATLAB implementation.
Cardiac and respiratory motion extraction for MRI using Pilot Tone-a patient study
Jan 31, 2022Abstract:Background:The Pilot Tone (PT) technology allows contactless monitoring of physiological motion during the MRI scan. Several studies have shown that both respiratory and cardiac motion can be extracted from the PT signal successfully. However, most of these studies were performed in healthy volunteers. In this study, we seek to evaluate the accuracy and reliability of the cardiac and respiratory signals extracted from PT in patients clinically referred for cardiovascular MRI (CMR). Methods: Twenty-three patients were included in this study, each scanned under free-breathing conditions using a balanced steady-state free-precession real-time (RT) cine sequence on a 1.5T scanner. The PT signal was generated by a built-in PT transmitter integrated within the body array coil. For comparison, ECG and BioMatrix (BM) respiratory sensor signals were also synchronously recorded. To assess the performances of PT, ECG, and BM, cardiac and respiratory signals extracted from the RT cine images were used as the ground truth. Results: The respiratory motion extracted from PT correlated positively with the image-derived respiratory signal in all cases and showed a stronger correlation (absolute coefficient: 0.95-0.09) than BM (0.72-0.24). For the cardiac signal, the precision of PT-based triggers (standard deviation of PT trigger locations relative to ECG triggers) ranged from 6.6 to 81.2 ms (median 19.5 ms). Overall, the performance of PT-based trigger extraction was comparable to that of ECG. Conclusions: This study demonstrates the potential of PT to monitor both respiratory and cardiac motion in patients clinically referred for CMR.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge