Yohei Harada
Emulating Clinical Quality Muscle B-mode Ultrasound Images from Plane Wave Images Using a Two-Stage Machine Learning Model
Dec 07, 2024Abstract:Research ultrasound scanners such as the Verasonics Vantage often lack the advanced image processing algorithms used by clinical systems. Image quality is even lower in plane wave imaging - often used for shear wave elasticity imaging (SWEI) - which sacrifices spatial resolution for temporal resolution. As a result, delay-and-summed images acquired from SWEI have limited interpretability. In this project, a two-stage machine learning model was trained to enhance single plane wave images of muscle acquired with a Verasonics Vantage system. The first stage of the model consists of a U-Net trained to emulate plane wave compounding, histogram matching, and unsharp masking using paired images. The second stage consists of a CycleGAN trained to emulate clinical muscle B-modes using unpaired images. This two-stage model was implemented on the Verasonics Vantage research ultrasound scanner, and its ability to provide high-speed image formation at a frame rate of 28.5 +/- 0.6 FPS from a single plane wave transmit was demonstrated. A reader study with two physicians demonstrated that these processed images had significantly greater structural fidelity and less speckle than the original plane wave images.
A New Deep State-Space Analysis Framework for Patient Latent State Estimation and Classification from EHR Time Series Data
Jul 21, 2023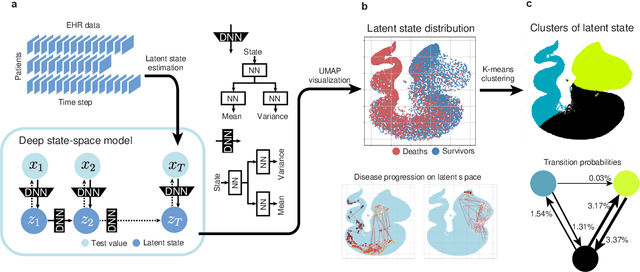
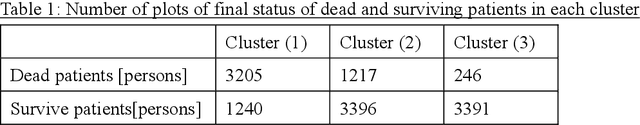
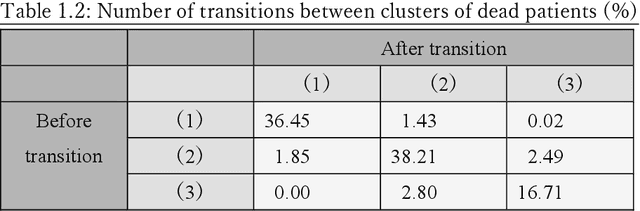
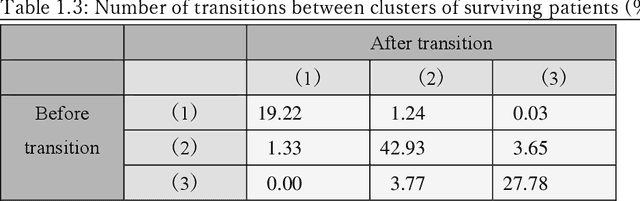
Abstract:Many diseases, including cancer and chronic conditions, require extended treatment periods and long-term strategies. Machine learning and AI research focusing on electronic health records (EHRs) have emerged to address this need. Effective treatment strategies involve more than capturing sequential changes in patient test values. It requires an explainable and clinically interpretable model by capturing the patient's internal state over time. In this study, we propose the "deep state-space analysis framework," using time-series unsupervised learning of EHRs with a deep state-space model. This framework enables learning, visualizing, and clustering of temporal changes in patient latent states related to disease progression. We evaluated our framework using time-series laboratory data from 12,695 cancer patients. By estimating latent states, we successfully discover latent states related to prognosis. By visualization and cluster analysis, the temporal transition of patient status and test items during state transitions characteristic of each anticancer drug were identified. Our framework surpasses existing methods in capturing interpretable latent space. It can be expected to enhance our comprehension of disease progression from EHRs, aiding treatment adjustments and prognostic determinations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge