Yeqi Wang
Adaptive PromptNet For Auxiliary Glioma Diagnosis without Contrast-Enhanced MRI
Nov 15, 2022Abstract:Multi-contrast magnetic resonance imaging (MRI)-based automatic auxiliary glioma diagnosis plays an important role in the clinic. Contrast-enhanced MRI sequences (e.g., contrast-enhanced T1-weighted imaging) were utilized in most of the existing relevant studies, in which remarkable diagnosis results have been reported. Nevertheless, acquiring contrast-enhanced MRI data is sometimes not feasible due to the patients physiological limitations. Furthermore, it is more time-consuming and costly to collect contrast-enhanced MRI data in the clinic. In this paper, we propose an adaptive PromptNet to address these issues. Specifically, a PromptNet for glioma grading utilizing only non-enhanced MRI data has been constructed. PromptNet receives constraints from features of contrast-enhanced MR data during training through a designed prompt loss. To further boost the performance, an adaptive strategy is designed to dynamically weight the prompt loss in a sample-based manner. As a result, PromptNet is capable of dealing with more difficult samples. The effectiveness of our method is evaluated on a widely-used BraTS2020 dataset, and competitive glioma grading performance on NE-MRI data is achieved.
Expert Knowledge-guided Geometric Representation Learning for Magnetic Resonance Imaging-based Glioma Grading
Jan 08, 2022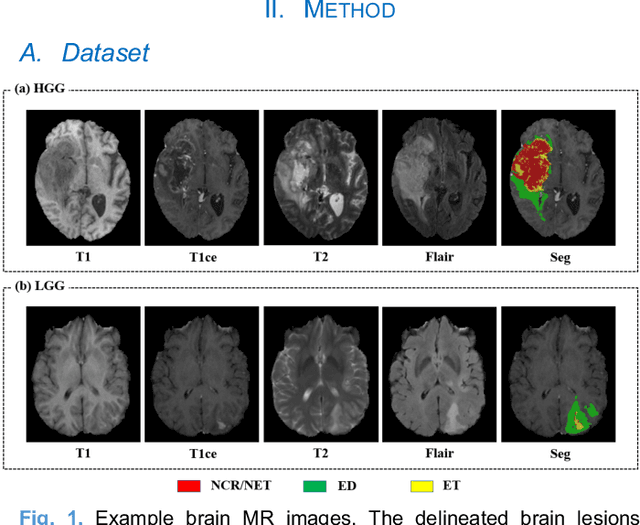
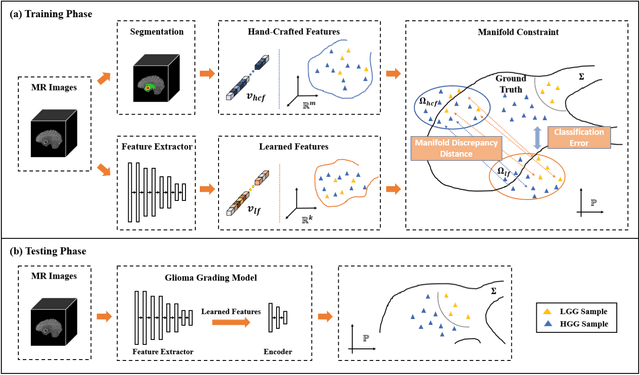
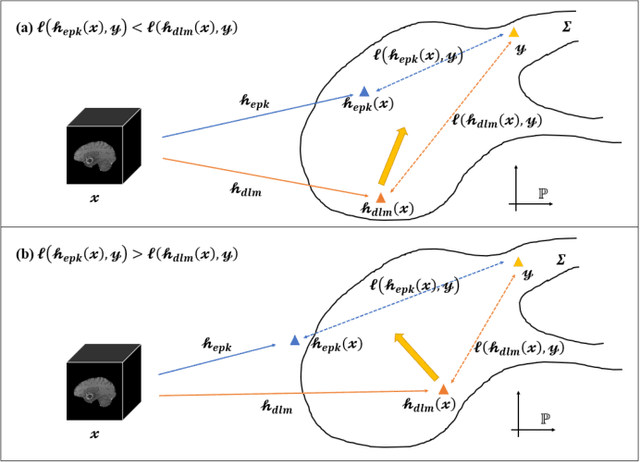
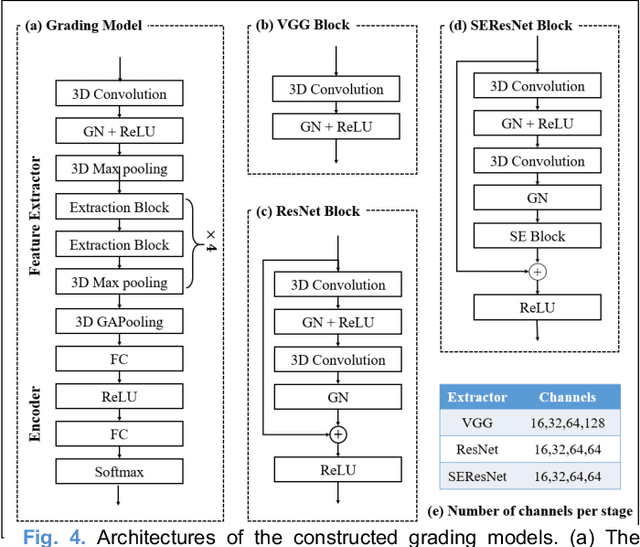
Abstract:Radiomics and deep learning have shown high popularity in automatic glioma grading. Radiomics can extract hand-crafted features that quantitatively describe the expert knowledge of glioma grades, and deep learning is powerful in extracting a large number of high-throughput features that facilitate the final classification. However, the performance of existing methods can still be improved as their complementary strengths have not been sufficiently investigated and integrated. Furthermore, lesion maps are usually needed for the final prediction at the testing phase, which is very troublesome. In this paper, we propose an expert knowledge-guided geometric representation learning (ENROL) framework . Geometric manifolds of hand-crafted features and learned features are constructed to mine the implicit relationship between deep learning and radiomics, and therefore to dig mutual consent and essential representation for the glioma grades. With a specially designed manifold discrepancy measurement, the grading model can exploit the input image data and expert knowledge more effectively in the training phase and get rid of the requirement of lesion segmentation maps at the testing phase. The proposed framework is flexible regarding deep learning architectures to be utilized. Three different architectures have been evaluated and five models have been compared, which show that our framework can always generate promising results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge