Wolfgang Weber
CHU Poitiers - Département de médecine nucléaire
RSV: Robotic Sonography for Thyroid Volumetry
Dec 13, 2021



Abstract:In nuclear medicine, radioiodine therapy is prescribed to treat diseases like hyperthyroidism. The calculation of the prescribed dose depends, amongst other factors, on the thyroid volume. This is currently estimated using conventional 2D ultrasound imaging. However, this modality is inherently user-dependant, resulting in high variability in volume estimations. To increase reproducibility and consistency, we uniquely combine a neural network-based segmentation with an automatic robotic ultrasound scanning for thyroid volumetry. The robotic acquisition is achieved by using a 6 DOF robotic arm with an attached ultrasound probe. Its movement is based on an online segmentation of each thyroid lobe and the appearance of the US image. During post-processing, the US images are segmented to obtain a volume estimation. In an ablation study, we demonstrated the superiority of the motion guidance algorithms for the robot arm movement compared to a naive linear motion, executed by the robot in terms of volumetric accuracy. In a user study on a phantom, we compared conventional 2D ultrasound measurements with our robotic system. The mean volume measurement error of ultrasound expert users could be significantly decreased from 20.85+/-16.10% to only 8.23+/-3.10% compared to the ground truth. This tendency was observed even more in non-expert users where the mean error improvement with the robotic system was measured to be as high as $85\%$ which clearly shows the advantages of the robotic support.
Tracked 3D Ultrasound and Deep Neural Network-based Thyroid Segmentation reduce Interobserver Variability in Thyroid Volumetry
Aug 10, 2021

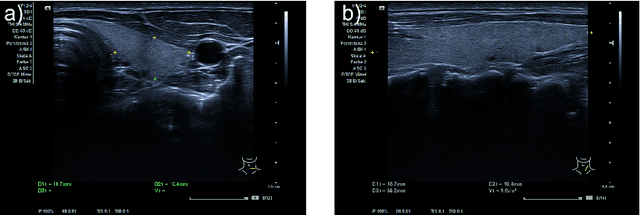
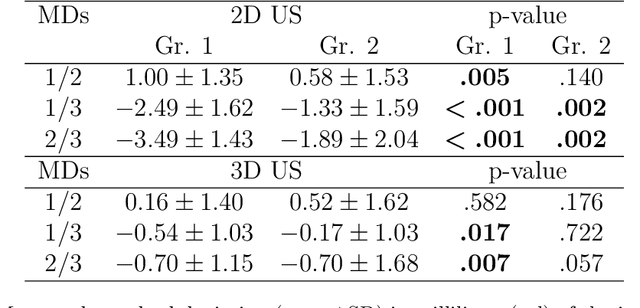
Abstract:Background: Thyroid volumetry is crucial in diagnosis, treatment and monitoring of thyroid diseases. However, conventional thyroid volumetry with 2D ultrasound is highly operator-dependent. This study compares 2D ultrasound and tracked 3D ultrasound with an automatic thyroid segmentation based on a deep neural network regarding inter- and intraobserver variability, time and accuracy. Volume reference was MRI. Methods: 28 healthy volunteers were scanned with 2D and 3D ultrasound as well as by MRI. Three physicians (MD 1, 2, 3) with different levels of experience (6, 4 and 1 a) performed three 2D ultrasound and three tracked 3D ultrasound scans on each volunteer. In the 2D scans the thyroid lobe volumes were calculated with the ellipsoid formula. A convolutional deep neural network (CNN) segmented the 3D thyroid lobes automatically. On MRI (T1 VIBE sequence) the thyroid was manually segmented by an experienced medical doctor. Results: The CNN was trained to obtain a dice score of 0.94. The interobserver variability comparing two MDs showed mean differences for 2D and 3D respectively of 0.58 ml to 0.52 ml (MD1 vs. 2), -1.33 ml to -0.17 ml (MD1 vs. 3) and -1.89 ml to -0.70 ml (MD2 vs. 3). Paired samples t-tests showed significant differences in two comparisons for 2D and none for 3D. Intraobsever variability was similar for 2D and 3D ultrasound. Comparison of ultrasound volumes and MRI volumes by paired samples t-tests showed a significant difference for the 2D volumetry of all MDs, and no significant difference for 3D ultrasound. Acquisition time was significantly shorter for 3D ultrasound. Conclusion: Tracked 3D ultrasound combined with a CNN segmentation significantly reduces interobserver variability in thyroid volumetry and increases the accuracy of the measurements with shorter acquisition times.
End-to-End Learning-Based Ultrasound Reconstruction
Apr 09, 2019


Abstract:Ultrasound imaging is caught between the quest for the highest image quality, and the necessity for clinical usability. Our contribution is two-fold: First, we propose a novel fully convolutional neural network for ultrasound reconstruction. Second, a custom loss function tailored to the modality is employed for end-to-end training of the network. We demonstrate that training a network to map time-delayed raw data to a minimum variance ground truth offers performance increases in a clinical environment. In doing so, a path is explored towards improved clinically viable ultrasound reconstruction. The proposed method displays both promising image reconstruction quality and acquisition frequency when integrated for live ultrasound scanning. A clinical evaluation is conducted to verify the diagnostic usefulness of the proposed method in a clinical setting.
Reliability of PET/CT shape and heterogeneity features in functional and morphological components of Non-Small Cell Lung Cancer tumors: a repeatability analysis in a prospective multi-center cohort
Oct 05, 2016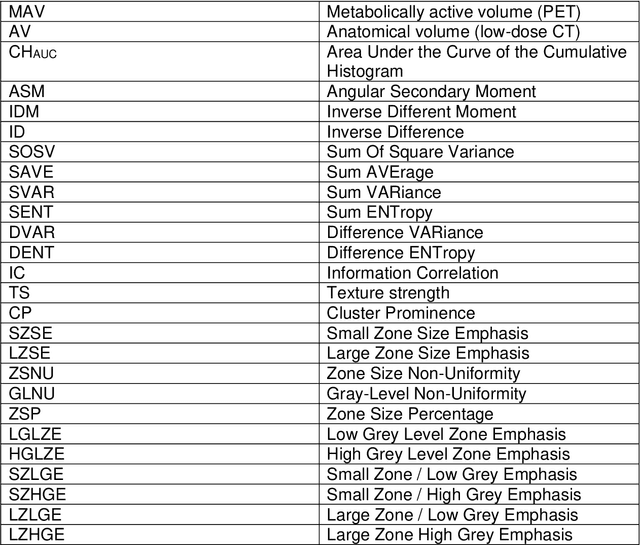
Abstract:Purpose: The main purpose of this study was to assess the reliability of shape and heterogeneity features in both Positron Emission Tomography (PET) and low-dose Computed Tomography (CT) components of PET/CT. A secondary objective was to investigate the impact of image quantization.Material and methods: A Health Insurance Portability and Accountability Act -compliant secondary analysis of deidentified prospectively acquired PET/CT test-retest datasets of 74 patients from multi-center Merck and ACRIN trials was performed. Metabolically active volumes were automatically delineated on PET with Fuzzy Locally Adaptive Bayesian algorithm. 3DSlicerTM was used to semi-automatically delineate the anatomical volumes on low-dose CT components. Two quantization methods were considered: a quantization into a set number of bins (quantizationB) and an alternative quantization with bins of fixed width (quantizationW). Four shape descriptors, ten first-order metrics and 26 textural features were computed. Bland-Altman analysis was used to quantify repeatability. Features were subsequently categorized as very reliable, reliable, moderately reliable and poorly reliable with respect to the corresponding volume variability. Results: Repeatability was highly variable amongst features. Numerous metrics were identified as poorly or moderately reliable. Others were (very) reliable in both modalities, and in all categories (shape, 1st-, 2nd- and 3rd-order metrics). Image quantization played a major role in the features repeatability. Features were more reliable in PET with quantizationB, whereas quantizationW showed better results in CT.Conclusion: The test-retest repeatability of shape and heterogeneity features in PET and low-dose CT varied greatly amongst metrics. The level of repeatability also depended strongly on the quantization step, with different optimal choices for each modality. The repeatability of PET and low-dose CT features should be carefully taken into account when selecting metrics to build multiparametric models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge