Wenbin Shi
Uncertainty-Based Biological Age Estimation of Brain MRI Scans
Mar 15, 2021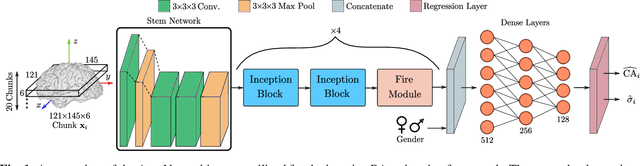
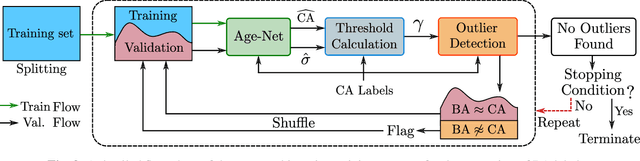
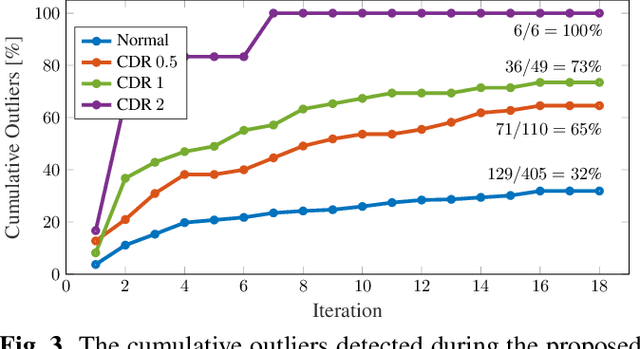
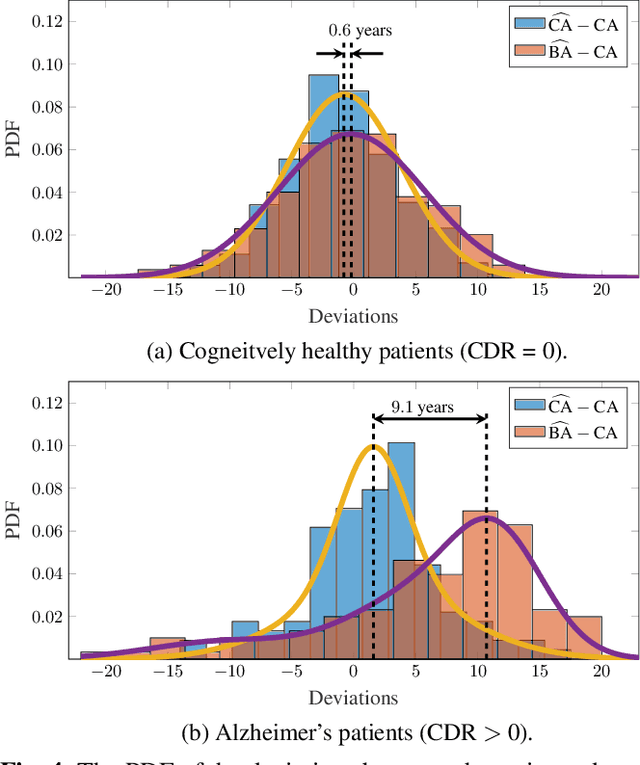
Abstract:Age is an essential factor in modern diagnostic procedures. However, assessment of the true biological age (BA) remains a daunting task due to the lack of reference ground-truth labels. Current BA estimation approaches are either restricted to skeletal images or rely on non-imaging modalities that yield a whole-body BA assessment. However, various organ systems may exhibit different aging characteristics due to lifestyle and genetic factors. In this initial study, we propose a new framework for organ-specific BA estimation utilizing 3D magnetic resonance image (MRI) scans. As a first step, this framework predicts the chronological age (CA) together with the corresponding patient-dependent aleatoric uncertainty. An iterative training algorithm is then utilized to segregate atypical aging patients from the given population based on the predicted uncertainty scores. In this manner, we hypothesize that training a new model on the remaining population should approximate the true BA behavior. We apply the proposed methodology on a brain MRI dataset containing healthy individuals as well as Alzheimer's patients. We demonstrate the correlation between the predicted BAs and the expected cognitive deterioration in Alzheimer's patients.
Age-Net: An MRI-Based Iterative Framework for Biological Age Estimation
Sep 22, 2020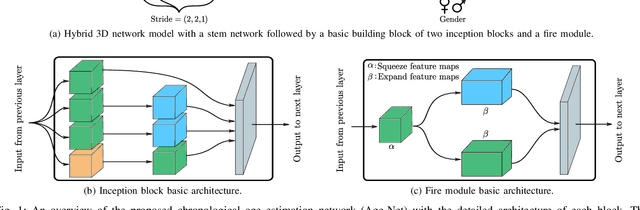
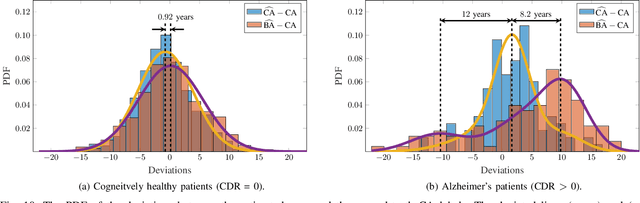
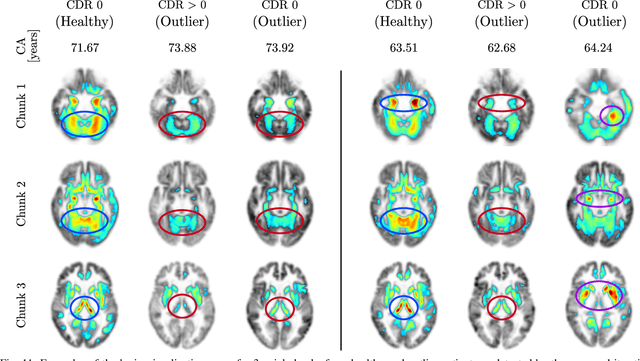
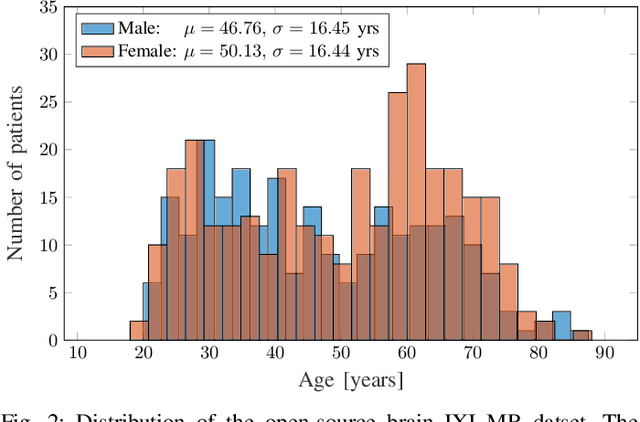
Abstract:The concept of biological age (BA) - although important in clinical practice - is hard to grasp mainly due to lack of a clearly defined reference standard. For specific applications, especially in pediatrics, medical image data are used for BA estimation in a routine clinical context. Beyond this young age group, BA estimation is restricted to whole-body assessment using non-imaging indicators such as blood biomarkers, genetic and cellular data. However, various organ systems may exhibit different aging characteristics due to lifestyle and genetic factors. Thus, a whole-body assessment of the BA does not reflect the deviations of aging behavior between organs. To this end, we propose a new imaging-based framework for organ-specific BA estimation. As a first step, we introduce a chronological age (CA) estimation framework using deep convolutional neural networks (Age-Net). We quantitatively assess the performance of this framework in comparison to existing CA estimation approaches. Furthermore, we expand upon Age-Net with a novel iterative data-cleaning algorithm to segregate atypical-aging patients (BA $\not \approx$ CA) from the given population. In this manner, we hypothesize that the remaining population should approximate the true BA behaviour. For this initial study, we apply the proposed methodology on a brain magnetic resonance image (MRI) dataset containing healthy individuals as well as Alzheimer's patients with different dementia ratings. We demonstrate the correlation between the predicted BAs and the expected cognitive deterioration in Alzheimer's patients. A statistical and visualization-based analysis has provided evidence regarding the potential and current challenges of the proposed methodology.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge