Vivek Prabhakaran
Stroke Lesion Segmentation using Multi-Stage Cross-Scale Attention
Jan 26, 2025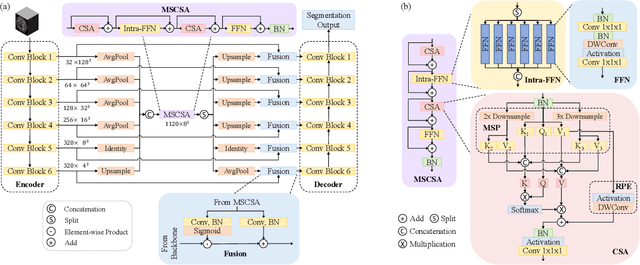
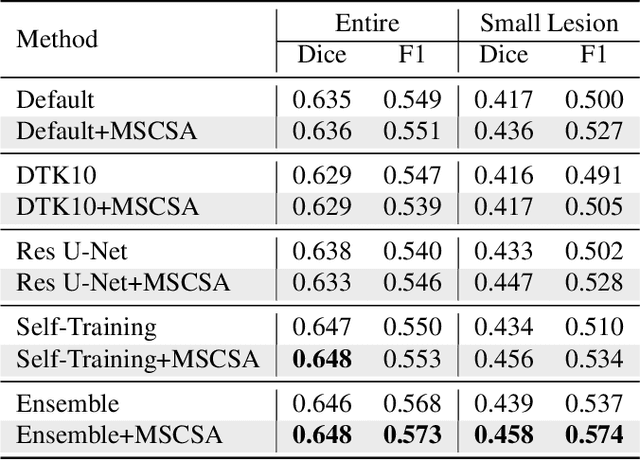
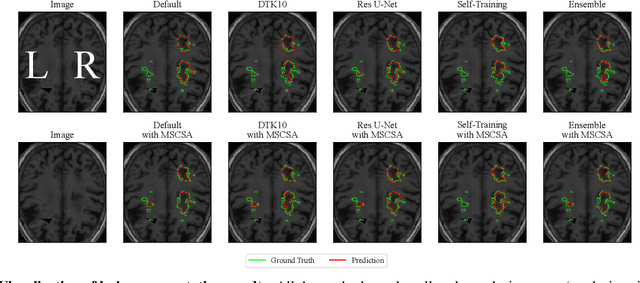
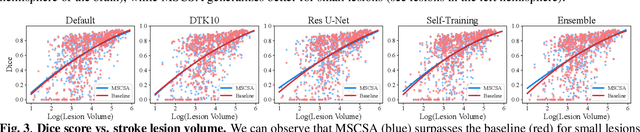
Abstract:Precise characterization of stroke lesions from MRI data has immense value in prognosticating clinical and cognitive outcomes following a stroke. Manual stroke lesion segmentation is time-consuming and requires the expertise of neurologists and neuroradiologists. Often, lesions are grossly characterized for their location and overall extent using bounding boxes without specific delineation of their boundaries. While such characterization provides some clinical value, to develop a precise mechanistic understanding of the impact of lesions on post-stroke vascular contributions to cognitive impairments and dementia (VCID), the stroke lesions need to be fully segmented with accurate boundaries. This work introduces the Multi-Stage Cross-Scale Attention (MSCSA) mechanism, applied to the U-Net family, to improve the mapping between brain structural features and lesions of varying sizes. Using the Anatomical Tracings of Lesions After Stroke (ATLAS) v2.0 dataset, MSCSA outperforms all baseline methods in both Dice and F1 scores on a subset focusing on small lesions, while maintaining competitive performance across the entire dataset. Notably, the ensemble strategy incorporating MSCSA achieves the highest scores for Dice and F1 on both the full dataset and the small lesion subset. These results demonstrate the effectiveness of MSCSA in segmenting small lesions and highlight its robustness across different training schemes for large stroke lesions. Our code is available at: https://github.com/nadluru/StrokeLesSeg.
Segmenting Small Stroke Lesions with Novel Labeling Strategies
Aug 06, 2024Abstract:Deep neural networks have demonstrated exceptional efficacy in stroke lesion segmentation. However, the delineation of small lesions, critical for stroke diagnosis, remains a challenge. In this study, we propose two straightforward yet powerful approaches that can be seamlessly integrated into a variety of networks: Multi-Size Labeling (MSL) and Distance-Based Labeling (DBL), with the aim of enhancing the segmentation accuracy of small lesions. MSL divides lesion masks into various categories based on lesion volume while DBL emphasizes the lesion boundaries. Experimental evaluations on the Anatomical Tracings of Lesions After Stroke (ATLAS) v2.0 dataset showcase that an ensemble of MSL and DBL achieves consistently better or equal performance on recall (3.6% and 3.7%), F1 (2.4% and 1.5%), and Dice scores (1.3% and 0.0%) compared to the top-1 winner of the 2022 MICCAI ATLAS Challenge on both the subset only containing small lesions and the entire dataset, respectively. Notably, on the mini-lesion subset, a single MSL model surpasses the previous best ensemble strategy, with enhancements of 1.0% and 0.3% on F1 and Dice scores, respectively. Our code is available at: https://github.com/nadluru/StrokeLesSeg.
DUAL-GLOW: Conditional Flow-Based Generative Model for Modality Transfer
Aug 21, 2019



Abstract:Positron emission tomography (PET) imaging is an imaging modality for diagnosing a number of neurological diseases. In contrast to Magnetic Resonance Imaging (MRI), PET is costly and involves injecting a radioactive substance into the patient. Motivated by developments in modality transfer in vision, we study the generation of certain types of PET images from MRI data. We derive new flow-based generative models which we show perform well in this small sample size regime (much smaller than dataset sizes available in standard vision tasks). Our formulation, DUAL-GLOW, is based on two invertible networks and a relation network that maps the latent spaces to each other. We discuss how given the prior distribution, learning the conditional distribution of PET given the MRI image reduces to obtaining the conditional distribution between the two latent codes w.r.t. the two image types. We also extend our framework to leverage 'side' information (or attributes) when available. By controlling the PET generation through 'conditioning' on age, our model is also able to capture brain FDG-PET (hypometabolism) changes, as a function of age. We present experiments on the Alzheimers Disease Neuroimaging Initiative (ADNI) dataset with 826 subjects, and obtain good performance in PET image synthesis, qualitatively and quantitatively better than recent works.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge