Troy Long
Accurate Real Time Localization Tracking in A Clinical Environment using Bluetooth Low Energy and Deep Learning
Oct 15, 2018



Abstract:Deep learning has started to revolutionize several different industries, and the applications of these methods in medicine are now becoming more commonplace. This study focuses on investigating the feasibility of tracking patients and clinical staff wearing Bluetooth Low Energy (BLE) tags in a radiation oncology clinic using artificial neural networks (ANNs) and convolutional neural networks (CNNs). The performance of these networks was compared to relative received signal strength indicator (RSSI) thresholding and triangulation. By utilizing temporal information, a combined CNN+ANN network was capable of correctly identifying the location of the BLE tag with an accuracy of 99.9%. It outperformed a CNN model (accuracy = 94%), a thresholding model employing majority voting (accuracy = 95%), and a triangulation classifier utilizing majority voting (accuracy = 95%). Future studies will seek to deploy this affordable real time location system in hospitals to improve clinical workflow, efficiency, and patient safety.
Dose Prediction with U-net: A Feasibility Study for Predicting Dose Distributions from Contours using Deep Learning on Prostate IMRT Patients
May 23, 2018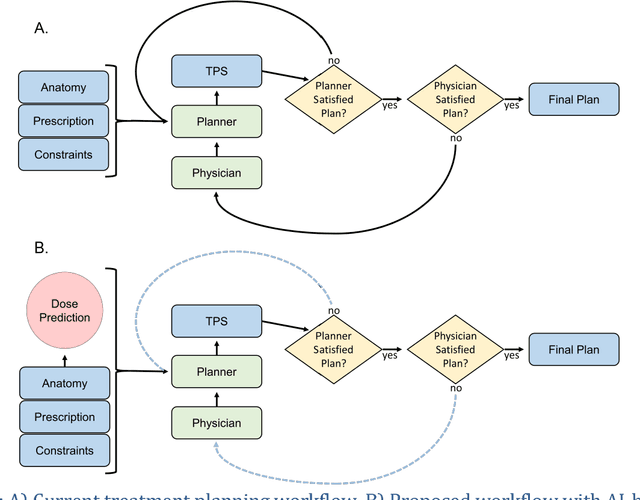
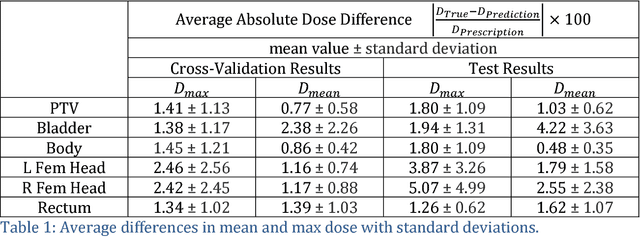
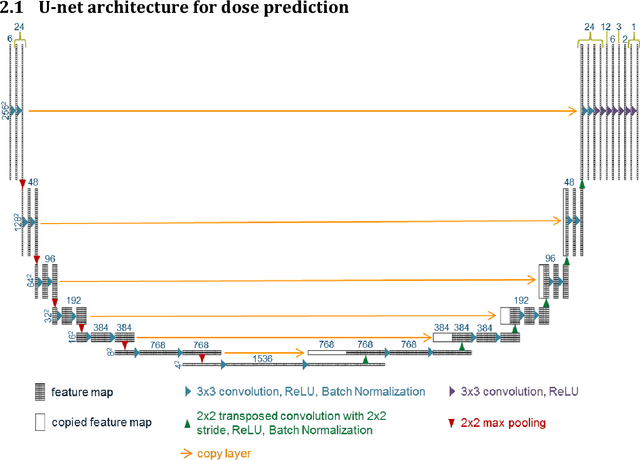
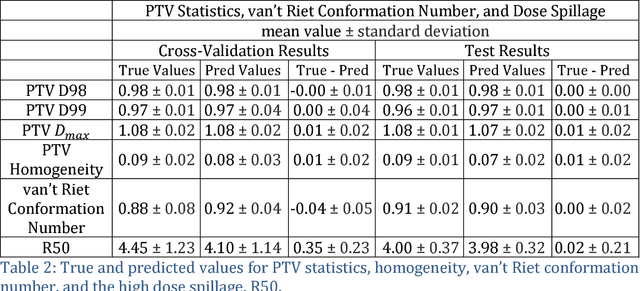
Abstract:With the advancement of treatment modalities in radiation therapy for cancer patients, outcomes have improved, but at the cost of increased treatment plan complexity and planning time. The accurate prediction of dose distributions would alleviate this issue by guiding clinical plan optimization to save time and maintain high quality plans. We have modified a convolutional deep network model, U-net (originally designed for segmentation purposes), for predicting dose from patient image contours. We show that, as an example, we are able to accurately predict the dose of intensity-modulated radiation therapy (IMRT) for prostate cancer patients, where the average dice similarity coefficient is 0.91 when comparing the predicted vs. true isodose volumes between 0% and 100% of the prescription dose. The average value of the absolute differences in [max, mean] dose is found to be under 5% of the prescription dose, specifically for each structure is [1.80%, 1.03%](PTV), [1.94%, 4.22%](Bladder), [1.80%, 0.48%](Body), [3.87%, 1.79%](L Femoral Head), [5.07%, 2.55%](R Femoral Head), and [1.26%, 1.62%](Rectum) of the prescription dose. We thus managed to map a desired radiation dose distribution from a patient's PTV and OAR contours. As an additional advantage, relatively little data was used in the techniques and models described in this paper.
Towards automated patient data cleaning using deep learning: A feasibility study on the standardization of organ labeling
Dec 30, 2017



Abstract:Data cleaning consumes about 80% of the time spent on data analysis for clinical research projects. This is a much bigger problem in the era of big data and machine learning in the field of medicine where large volumes of data are being generated. We report an initial effort towards automated patient data cleaning using deep learning: the standardization of organ labeling in radiation therapy. Organs are often labeled inconsistently at different institutions (sometimes even within the same institution) and at different time periods, which poses a problem for clinical research, especially for multi-institutional collaborative clinical research where the acquired patient data is not being used effectively. We developed a convolutional neural network (CNN) to automatically identify each organ in the CT image and then label it with the standardized nomenclature presented at AAPM Task Group 263. We tested this model on the CT images of 54 patients with prostate and 100 patients with head and neck cancer who previously received radiation therapy. The model achieved 100% accuracy in detecting organs and assigning standardized labels for the patients tested. This work shows the feasibility of using deep learning in patient data cleaning that enables standardized datasets to be generated for effective intra- and interinstitutional collaborative clinical research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge