Theodore D. Satterthwaite
Concurrence: A dependence criterion for time series, applied to biological data
Dec 17, 2025Abstract:Measuring the statistical dependence between observed signals is a primary tool for scientific discovery. However, biological systems often exhibit complex non-linear interactions that currently cannot be captured without a priori knowledge or large datasets. We introduce a criterion for dependence, whereby two time series are deemed dependent if one can construct a classifier that distinguishes between temporally aligned vs. misaligned segments extracted from them. We show that this criterion, concurrence, is theoretically linked with dependence, and can become a standard approach for scientific analyses across disciplines, as it can expose relationships across a wide spectrum of signals (fMRI, physiological and behavioral data) without ad-hoc parameter tuning or large amounts of data.
Medical Image Harmonization Using Deep Learning Based Canonical Mapping: Toward Robust and Generalizable Learning in Imaging
Oct 11, 2020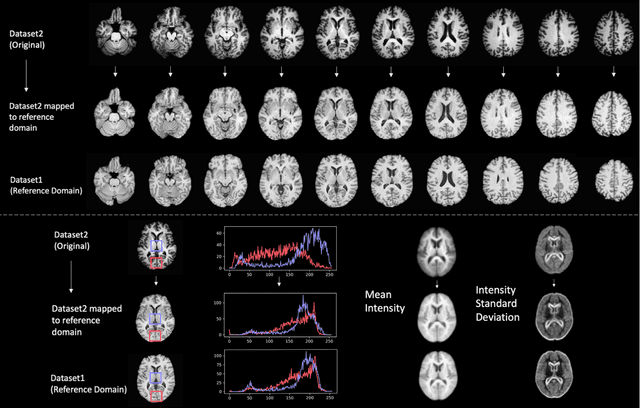
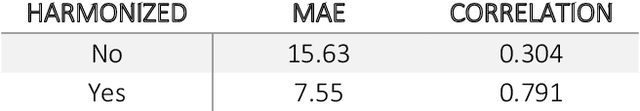
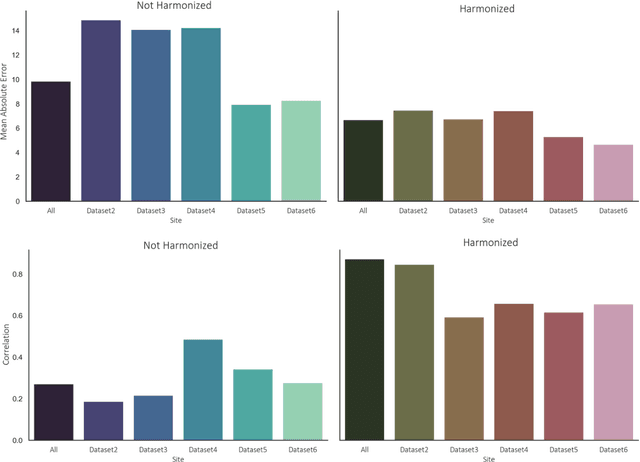
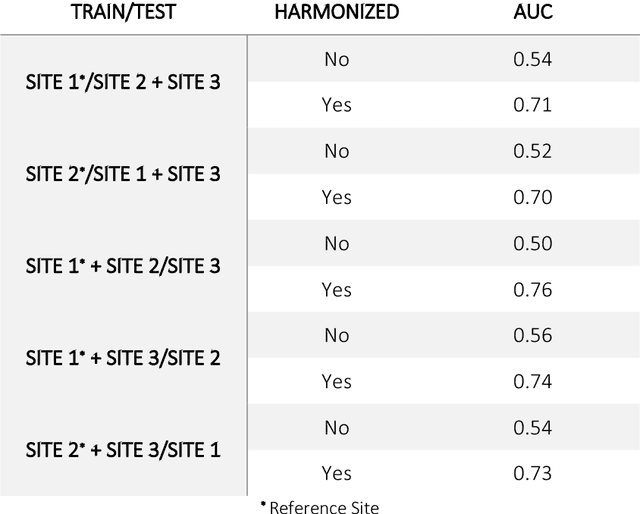
Abstract:Conventional and deep learning-based methods have shown great potential in the medical imaging domain, as means for deriving diagnostic, prognostic, and predictive biomarkers, and by contributing to precision medicine. However, these methods have yet to see widespread clinical adoption, in part due to limited generalization performance across various imaging devices, acquisition protocols, and patient populations. In this work, we propose a new paradigm in which data from a diverse range of acquisition conditions are "harmonized" to a common reference domain, where accurate model learning and prediction can take place. By learning an unsupervised image to image canonical mapping from diverse datasets to a reference domain using generative deep learning models, we aim to reduce confounding data variation while preserving semantic information, thereby rendering the learning task easier in the reference domain. We test this approach on two example problems, namely MRI-based brain age prediction and classification of schizophrenia, leveraging pooled cohorts of neuroimaging MRI data spanning 9 sites and 9701 subjects. Our results indicate a substantial improvement in these tasks in out-of-sample data, even when training is restricted to a single site.
Finding the needle in high-dimensional haystack: A tutorial on canonical correlation analysis
Dec 06, 2018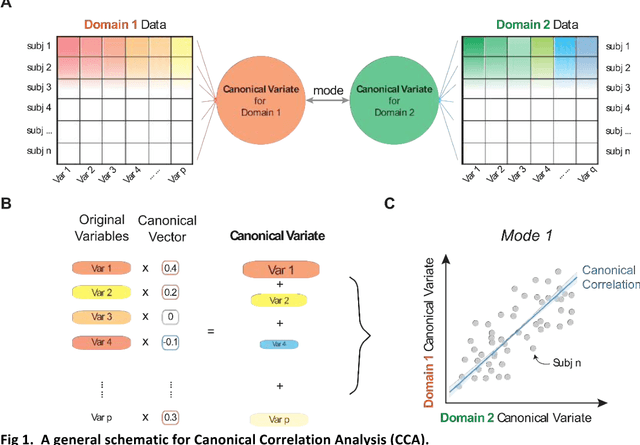
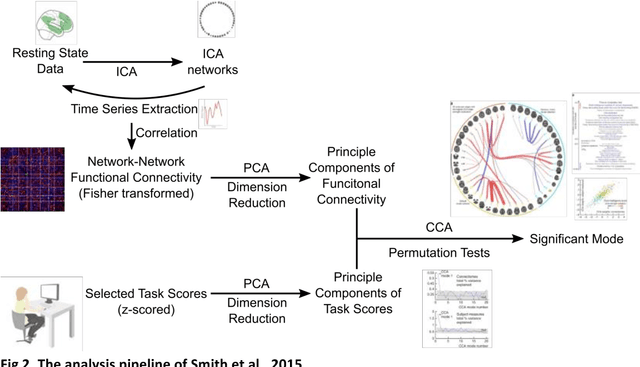
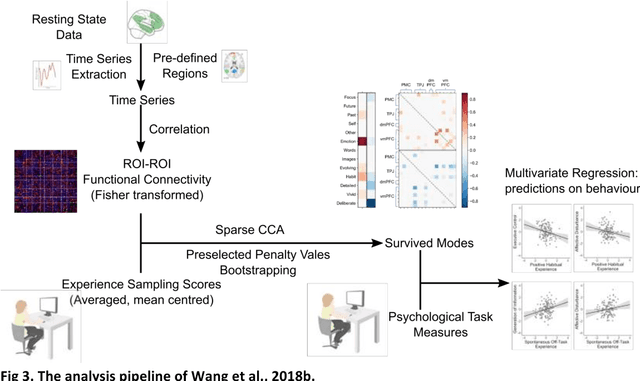
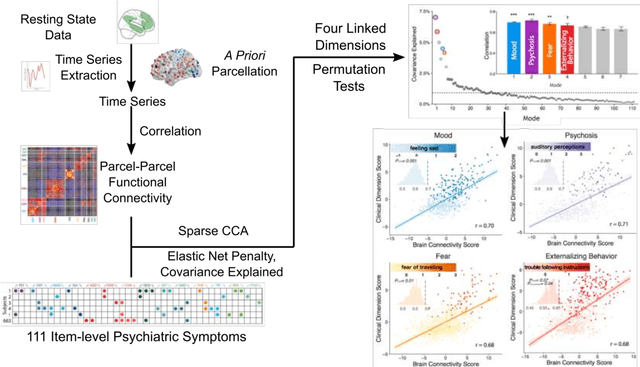
Abstract:Since the beginning of the 21st century, the size, breadth, and granularity of data in biology and medicine has grown rapidly. In the example of neuroscience, studies with thousands of subjects are becoming more common, which provide extensive phenotyping on the behavioral, neural, and genomic level with hundreds of variables. The complexity of such big data repositories offer new opportunities and pose new challenges to investigate brain, cognition, and disease. Canonical correlation analysis (CCA) is a prototypical family of methods for wrestling with and harvesting insight from such rich datasets. This doubly-multivariate tool can simultaneously consider two variable sets from different modalities to uncover essential hidden associations. Our primer discusses the rationale, promises, and pitfalls of CCA in biomedicine.
Brain Age Prediction Based on Resting-State Functional Connectivity Patterns Using Convolutional Neural Networks
Jan 11, 2018



Abstract:Brain age prediction based on neuroimaging data could help characterize both the typical brain development and neuropsychiatric disorders. Pattern recognition models built upon functional connectivity (FC) measures derived from resting state fMRI (rsfMRI) data have been successfully used to predict the brain age. However, most existing studies focus on coarse-grained FC measures between brain regions or intrinsic connectivity networks (ICNs), which may sacrifice fine-grained FC information of the rsfMRI data. Whole brain voxel-wise FC measures could provide fine-grained FC information of the brain and may improve the prediction performance. In this study, we develop a deep learning method to use convolutional neural networks (CNNs) to learn informative features from the fine-grained whole brain FC measures for the brain age prediction. Experimental results on a large dataset of resting-state fMRI demonstrate that the deep learning model with fine-grained FC measures could better predict the brain age.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge