Shaheer U Saeed
A Recycling Training Strategy for Medical Image Segmentation with Diffusion Denoising Models
Aug 30, 2023



Abstract:Denoising diffusion models have found applications in image segmentation by generating segmented masks conditioned on images. Existing studies predominantly focus on adjusting model architecture or improving inference such as test-time sampling strategies. In this work, we focus on training strategy improvements and propose a novel recycling method. During each training step, a segmentation mask is first predicted given an image and a random noise. This predicted mask, replacing the conventional ground truth mask, is used for denoising task during training. This approach can be interpreted as aligning the training strategy with inference by eliminating the dependence on ground truth masks for generating noisy samples. Our proposed method significantly outperforms standard diffusion training, self-conditioning, and existing recycling strategies across multiple medical imaging data sets: muscle ultrasound, abdominal CT, prostate MR, and brain MR. This holds true for two widely adopted sampling strategies: denoising diffusion probabilistic model and denoising diffusion implicit model. Importantly, existing diffusion models often display a declining or unstable performance during inference, whereas our novel recycling consistently enhances or maintains performance. Furthermore, we show for the first time that, under a fair comparison with the same network architectures and computing budget, the proposed recycling-based diffusion models achieved on-par performance with non-diffusion-based supervised training. This paper summarises these quantitative results and discusses their values, with a fully reproducible JAX-based implementation, released at https://github.com/mathpluscode/ImgX-DiffSeg.
Development and evaluation of intraoperative ultrasound segmentation with negative image frames and multiple observer labels
Jul 28, 2021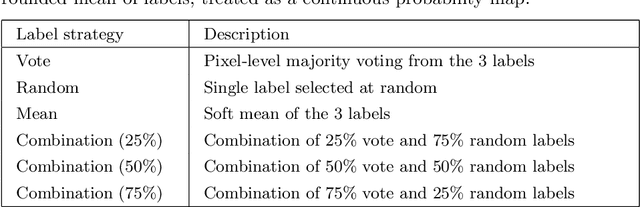
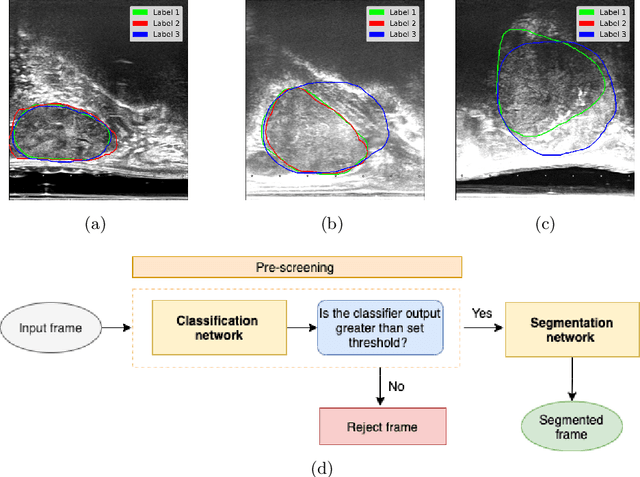
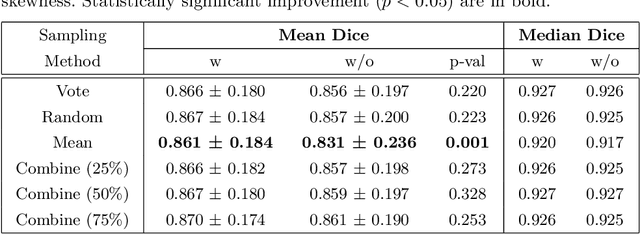
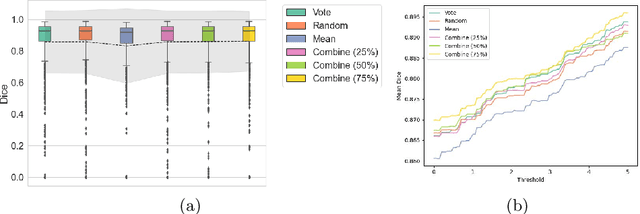
Abstract:When developing deep neural networks for segmenting intraoperative ultrasound images, several practical issues are encountered frequently, such as the presence of ultrasound frames that do not contain regions of interest and the high variance in ground-truth labels. In this study, we evaluate the utility of a pre-screening classification network prior to the segmentation network. Experimental results demonstrate that such a classifier, minimising frame classification errors, was able to directly impact the number of false positive and false negative frames. Importantly, the segmentation accuracy on the classifier-selected frames, that would be segmented, remains comparable to or better than those from standalone segmentation networks. Interestingly, the efficacy of the pre-screening classifier was affected by the sampling methods for training labels from multiple observers, a seemingly independent problem. We show experimentally that a previously proposed approach, combining random sampling and consensus labels, may need to be adapted to perform well in our application. Furthermore, this work aims to share practical experience in developing a machine learning application that assists highly variable interventional imaging for prostate cancer patients, to present robust and reproducible open-source implementations, and to report a set of comprehensive results and analysis comparing these practical, yet important, options in a real-world clinical application.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge