Iani JMB Gayo
Strategising template-guided needle placement for MR-targeted prostate biopsy
Jul 21, 2022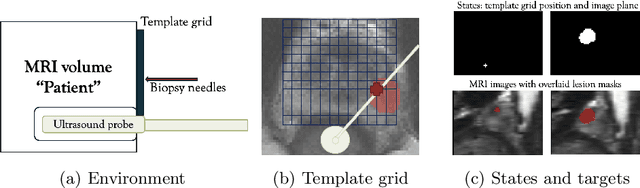
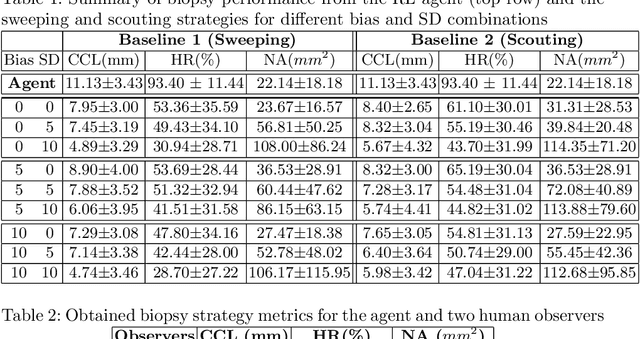
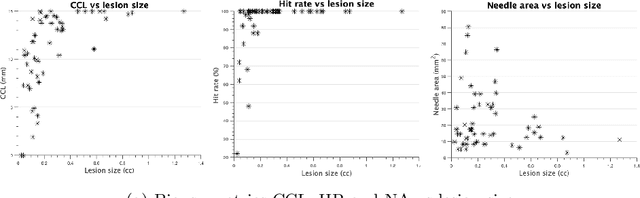
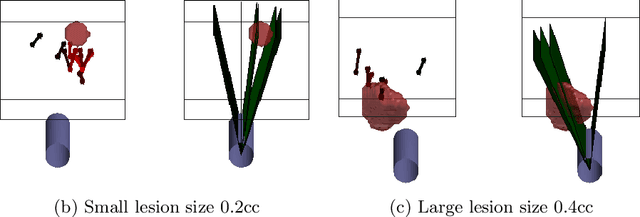
Abstract:Clinically significant prostate cancer has a better chance to be sampled during ultrasound-guided biopsy procedures, if suspected lesions found in pre-operative magnetic resonance (MR) images are used as targets. However, the diagnostic accuracy of the biopsy procedure is limited by the operator-dependent skills and experience in sampling the targets, a sequential decision making process that involves navigating an ultrasound probe and placing a series of sampling needles for potentially multiple targets. This work aims to learn a reinforcement learning (RL) policy that optimises the actions of continuous positioning of 2D ultrasound views and biopsy needles with respect to a guiding template, such that the MR targets can be sampled efficiently and sufficiently. We first formulate the task as a Markov decision process (MDP) and construct an environment that allows the targeting actions to be performed virtually for individual patients, based on their anatomy and lesions derived from MR images. A patient-specific policy can thus be optimised, before each biopsy procedure, by rewarding positive sampling in the MDP environment. Experiment results from fifty four prostate cancer patients show that the proposed RL-learned policies obtained a mean hit rate of 93% and an average cancer core length of 11 mm, which compared favourably to two alternative baseline strategies designed by humans, without hand-engineered rewards that directly maximise these clinically relevant metrics. Perhaps more interestingly, it is found that the RL agents learned strategies that were adaptive to the lesion size, where spread of the needles was prioritised for smaller lesions. Such a strategy has not been previously reported or commonly adopted in clinical practice, but led to an overall superior targeting performance when compared with intuitively designed strategies.
Development and evaluation of intraoperative ultrasound segmentation with negative image frames and multiple observer labels
Jul 28, 2021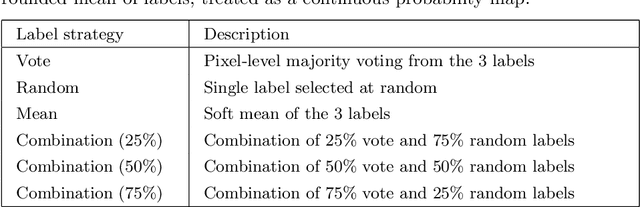
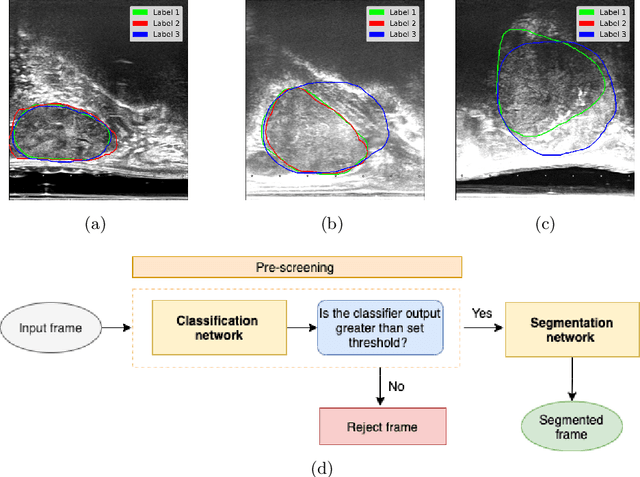
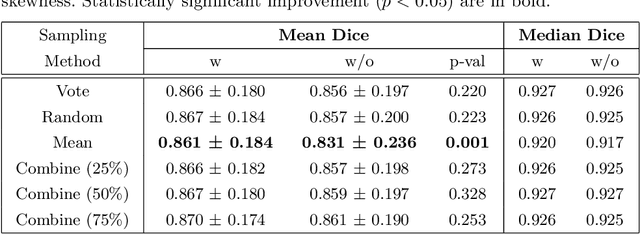
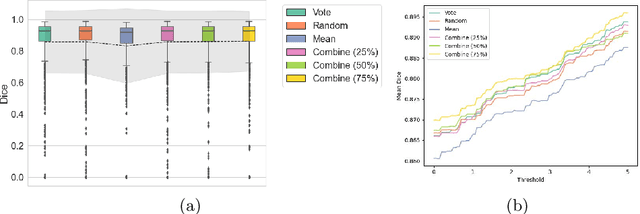
Abstract:When developing deep neural networks for segmenting intraoperative ultrasound images, several practical issues are encountered frequently, such as the presence of ultrasound frames that do not contain regions of interest and the high variance in ground-truth labels. In this study, we evaluate the utility of a pre-screening classification network prior to the segmentation network. Experimental results demonstrate that such a classifier, minimising frame classification errors, was able to directly impact the number of false positive and false negative frames. Importantly, the segmentation accuracy on the classifier-selected frames, that would be segmented, remains comparable to or better than those from standalone segmentation networks. Interestingly, the efficacy of the pre-screening classifier was affected by the sampling methods for training labels from multiple observers, a seemingly independent problem. We show experimentally that a previously proposed approach, combining random sampling and consensus labels, may need to be adapted to perform well in our application. Furthermore, this work aims to share practical experience in developing a machine learning application that assists highly variable interventional imaging for prostate cancer patients, to present robust and reproducible open-source implementations, and to report a set of comprehensive results and analysis comparing these practical, yet important, options in a real-world clinical application.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge