Roger G. Mark
Interpretable Machine Learning Model for Early Prediction of Mortality in Elderly Patients with Multiple Organ Dysfunction Syndrome (MODS): a Multicenter Retrospective Study and Cross Validation
Jan 28, 2020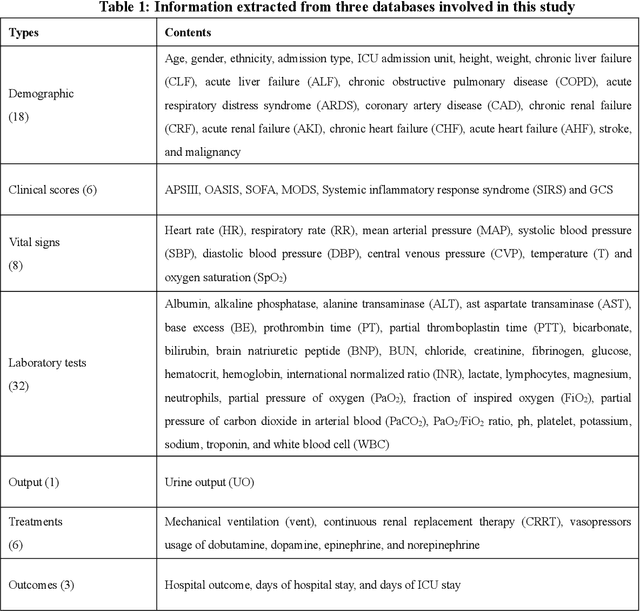
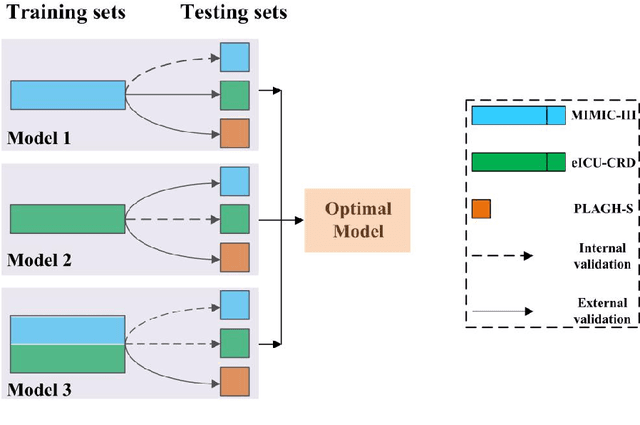
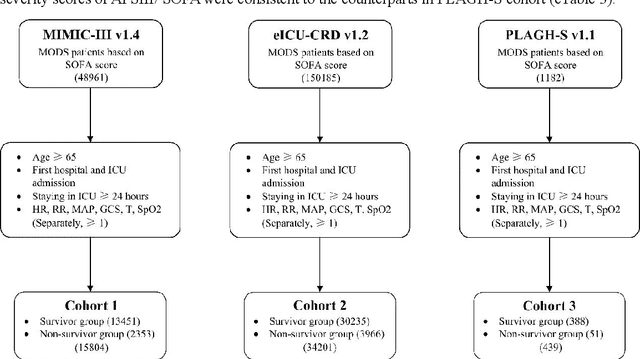
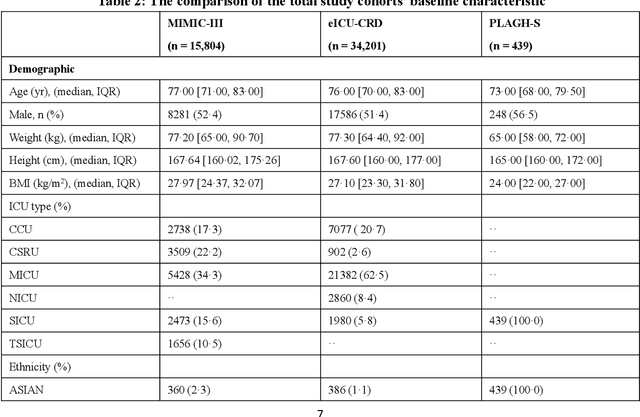
Abstract:Background: Elderly patients with MODS have high risk of death and poor prognosis. The performance of current scoring systems assessing the severity of MODS and its mortality remains unsatisfactory. This study aims to develop an interpretable and generalizable model for early mortality prediction in elderly patients with MODS. Methods: The MIMIC-III, eICU-CRD and PLAGH-S databases were employed for model generation and evaluation. We used the eXtreme Gradient Boosting model with the SHapley Additive exPlanations method to conduct early and interpretable predictions of patients' hospital outcome. Three types of data source combinations and five typical evaluation indexes were adopted to develop a generalizable model. Findings: The interpretable model, with optimal performance developed by using MIMIC-III and eICU-CRD datasets, was separately validated in MIMIC-III, eICU-CRD and PLAGH-S datasets (no overlapping with training set). The performances of the model in predicting hospital mortality as validated by the three datasets were: AUC of 0.858, sensitivity of 0.834 and specificity of 0.705; AUC of 0.849, sensitivity of 0.763 and specificity of 0.784; and AUC of 0.838, sensitivity of 0.882 and specificity of 0.691, respectively. Comparisons of AUC between this model and baseline models with MIMIC-III dataset validation showed superior performances of this model; In addition, comparisons in AUC between this model and commonly used clinical scores showed significantly better performance of this model. Interpretation: The interpretable machine learning model developed in this study using fused datasets with large sample sizes was robust and generalizable. This model outperformed the baseline models and several clinical scores for early prediction of mortality in elderly ICU patients. The interpretative nature of this model provided clinicians with the ranking of mortality risk features.
MIMIC-CXR: A large publicly available database of labeled chest radiographs
Jan 23, 2019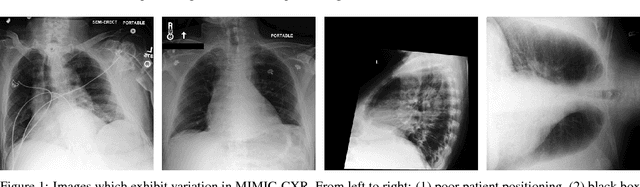
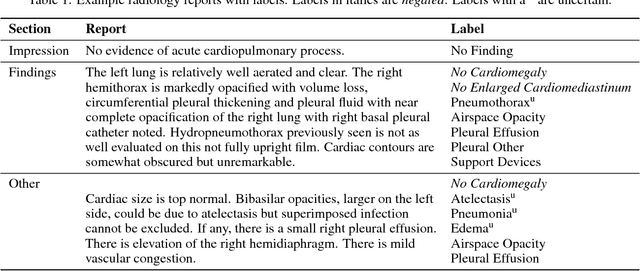
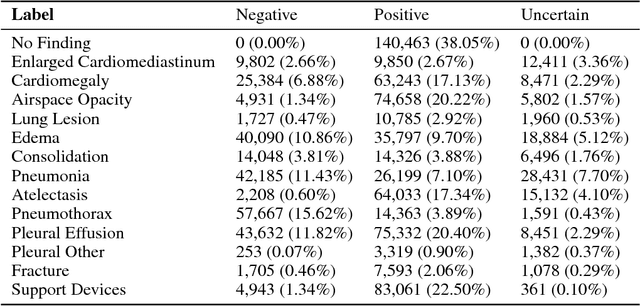
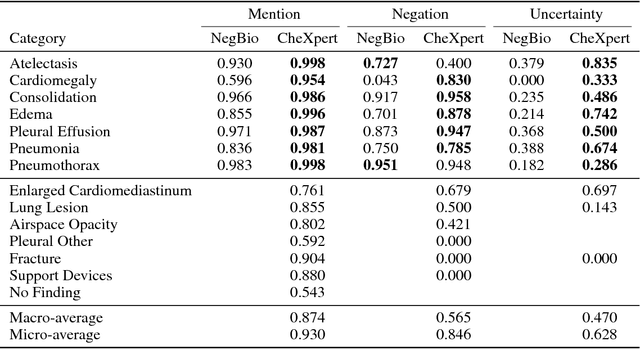
Abstract:Chest radiography is an extremely powerful imaging modality, allowing for a detailed inspection of a patient's thorax, but requiring specialized training for proper interpretation. With the advent of high performance general purpose computer vision algorithms, the accurate automated analysis of chest radiographs is becoming increasingly of interest to researchers. However, a key challenge in the development of these techniques is the lack of sufficient data. Here we describe MIMIC-CXR, a large dataset of 371,920 chest x-rays associated with 227,943 imaging studies sourced from the Beth Israel Deaconess Medical Center between 2011 - 2016. Each imaging study can pertain to one or more images, but most often are associated with two images: a frontal view and a lateral view. Images are provided with 14 labels derived from a natural language processing tool applied to the corresponding free-text radiology reports. All images have been de-identified to protect patient privacy. The dataset is made freely available to facilitate and encourage a wide range of research in medical computer vision.
False arrhythmia alarm reduction in the intensive care unit
Sep 11, 2017



Abstract:Research has shown that false alarms constitute more than 80% of the alarms triggered in the intensive care unit (ICU). The high false arrhythmia alarm rate has severe implications such as disruption of patient care, caregiver alarm fatigue, and desensitization from clinical staff to real life-threatening alarms. A method to reduce the false alarm rate would therefore greatly benefit patients as well as nurses in their ability to provide care. We here develop and describe a robust false arrhythmia alarm reduction system for use in the ICU. Building off of work previously described in the literature, we make use of signal processing and machine learning techniques to identify true and false alarms for five arrhythmia types. This baseline algorithm alone is able to perform remarkably well, with a sensitivity of 0.908, a specificity of 0.838, and a PhysioNet/CinC challenge score of 0.756. We additionally explore dynamic time warping techniques on both the entire alarm signal as well as on a beat-by-beat basis in an effort to improve performance of ventricular tachycardia, which has in the literature been one of the hardest arrhythmias to classify. Such an algorithm with strong performance and efficiency could potentially be translated for use in the ICU to promote overall patient care and recovery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge