Queenie Chan
Utilizing 3D Fast Spin Echo Anatomical Imaging to Reduce the Number of Contrast Preparations in $T_{1ρ}$ Quantification of Knee Cartilage Using Learning-Based Methods
Feb 13, 2025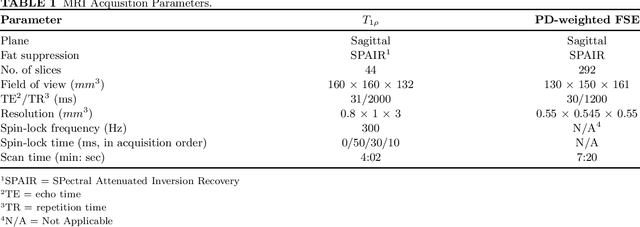
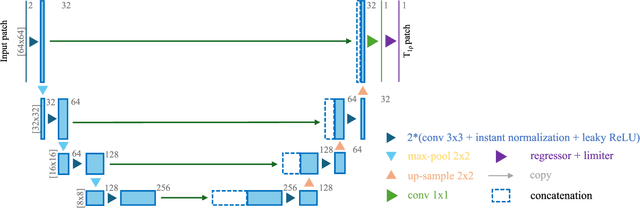
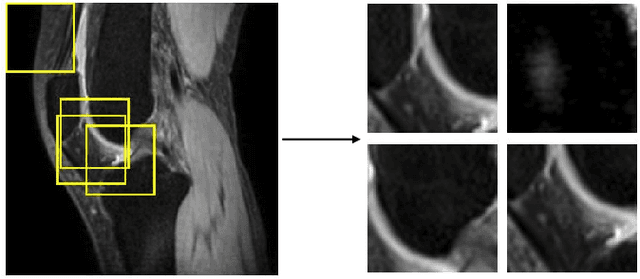
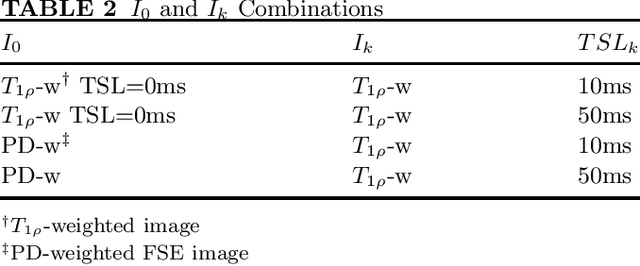
Abstract:Purpose: To propose and evaluate an accelerated $T_{1\rho}$ quantification method that combines $T_{1\rho}$-weighted fast spin echo (FSE) images and proton density (PD)-weighted anatomical FSE images, leveraging deep learning models for $T_{1\rho}$ mapping. The goal is to reduce scan time and facilitate integration into routine clinical workflows for osteoarthritis (OA) assessment. Methods: This retrospective study utilized MRI data from 40 participants (30 OA patients and 10 healthy volunteers). A volume of PD-weighted anatomical FSE images and a volume of $T_{1\rho}$-weighted images acquired at a non-zero spin-lock time were used as input to train deep learning models, including a 2D U-Net and a multi-layer perceptron (MLP). $T_{1\rho}$ maps generated by these models were compared with ground truth maps derived from a traditional non-linear least squares (NLLS) fitting method using four $T_{1\rho}$-weighted images. Evaluation metrics included mean absolute error (MAE), mean absolute percentage error (MAPE), regional error (RE), and regional percentage error (RPE). Results: Deep learning models achieved RPEs below 5% across all evaluated scenarios, outperforming NLLS methods, especially in low signal-to-noise conditions. The best results were obtained using the 2D U-Net, which effectively leveraged spatial information for accurate $T_{1\rho}$ fitting. The proposed method demonstrated compatibility with shorter TSLs, alleviating RF hardware and specific absorption rate (SAR) limitations. Conclusion: The proposed approach enables efficient $T_{1\rho}$ mapping using PD-weighted anatomical images, reducing scan time while maintaining clinical standards. This method has the potential to facilitate the integration of quantitative MRI techniques into routine clinical practice, benefiting OA diagnosis and monitoring.
Chemical Shift Encoding based Double Bonds Quantification in Triglycerides using Deep Image Prior
Jul 02, 2024



Abstract:This study evaluated a deep learning-based method using Deep Image Prior (DIP) to quantify triglyceride double bonds from chemical-shift encoded multi-echo gradient echo images without network training. We employed a cost function based on signal constraints to iteratively update the neural network on a single dataset. The method was validated using phantom experiments and in vivo scans. Results showed close alignment between measured and reference double bond values, with phantom experiments yielding a Pearson correlation coefficient of 0.96 (p = .0005). In vivo results demonstrated good agreement in subcutaneous fat. We conclude that Deep Image Prior shows feasibility for quantifying double bonds and fatty acid content from chemical-shift encoded multi-echo MRI.
An Uncertainty Aided Framework for Learning based Liver $T_1ρ$ Mapping and Analysis
Jul 07, 2023Abstract:Objective: Quantitative $T_1\rho$ imaging has potential for assessment of biochemical alterations of liver pathologies. Deep learning methods have been employed to accelerate quantitative $T_1\rho$ imaging. To employ artificial intelligence-based quantitative imaging methods in complicated clinical environment, it is valuable to estimate the uncertainty of the predicated $T_1\rho$ values to provide the confidence level of the quantification results. The uncertainty should also be utilized to aid the post-hoc quantitative analysis and model learning tasks. Approach: To address this need, we propose a parametric map refinement approach for learning-based $T_1\rho$ mapping and train the model in a probabilistic way to model the uncertainty. We also propose to utilize the uncertainty map to spatially weight the training of an improved $T_1\rho$ mapping network to further improve the mapping performance and to remove pixels with unreliable $T_1\rho$ values in the region of interest. The framework was tested on a dataset of 51 patients with different liver fibrosis stages. Main results: Our results indicate that the learning-based map refinement method leads to a relative mapping error of less than 3% and provides uncertainty estimation simultaneously. The estimated uncertainty reflects the actual error level, and it can be used to further reduce relative $T_1\rho$ mapping error to 2.60% as well as removing unreliable pixels in the region of interest effectively. Significance: Our studies demonstrate the proposed approach has potential to provide a learning-based quantitative MRI system for trustworthy $T_1\rho$ mapping of the liver.
Uncertainty-weighted Multi-tasking for $T_{1ρ}$ and T$_2$ Mapping in the Liver with Self-supervised Learning
Mar 14, 2023Abstract:Multi-parametric mapping of MRI relaxations in liver has the potential of revealing pathological information of the liver. A self-supervised learning based multi-parametric mapping method is proposed to map T$T_{1\rho}$ and T$_2$ simultaneously, by utilising the relaxation constraint in the learning process. Data noise of different mapping tasks is utilised to make the model uncertainty-aware, which adaptively weight different mapping tasks during learning. The method was examined on a dataset of 51 patients with non-alcoholic fatter liver disease. Results showed that the proposed method can produce comparable parametric maps to the traditional multi-contrast pixel wise fitting method, with a reduced number of images and less computation time. The uncertainty weighting also improves the model performance. It has the potential of accelerating MRI quantitative imaging.
Uncertainty-Aware Self-supervised Neural Network for Liver $T_{1ρ}$ Mapping with Relaxation Constraint
Jul 07, 2022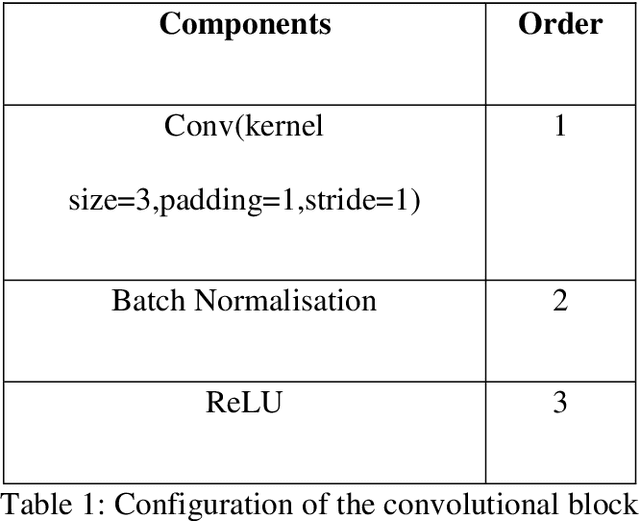

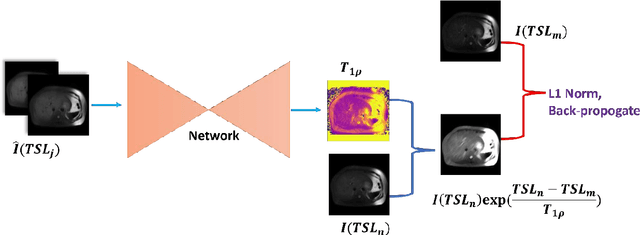
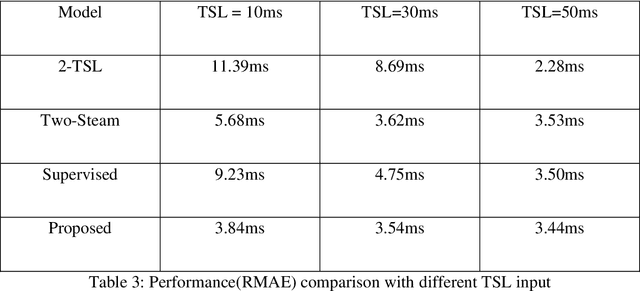
Abstract:$T_{1\rho}$ mapping is a promising quantitative MRI technique for the non-invasive assessment of tissue properties. Learning-based approaches can map $T_{1\rho}$ from a reduced number of $T_{1\rho}$ weighted images, but requires significant amounts of high quality training data. Moreover, existing methods do not provide the confidence level of the $T_{1\rho}$ estimation. To address these problems, we proposed a self-supervised learning neural network that learns a $T_{1\rho}$ mapping using the relaxation constraint in the learning process. Epistemic uncertainty and aleatoric uncertainty are modelled for the $T_{1\rho}$ quantification network to provide a Bayesian confidence estimation of the $T_{1\rho}$ mapping. The uncertainty estimation can also regularize the model to prevent it from learning imperfect data. We conducted experiments on $T_{1\rho}$ data collected from 52 patients with non-alcoholic fatty liver disease. The results showed that our method outperformed the existing methods for $T_{1\rho}$ quantification of the liver using as few as two $T_{1\rho}$-weighted images. Our uncertainty estimation provided a feasible way of modelling the confidence of the self-supervised learning based $T_{1\rho}$ estimation, which is consistent with the reality in liver $T_{1\rho}$ imaging.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge