Po-Yu Kao
Toward Drug-Target Interaction Prediction via Ensemble Modeling and Transfer Learning
Jul 28, 2021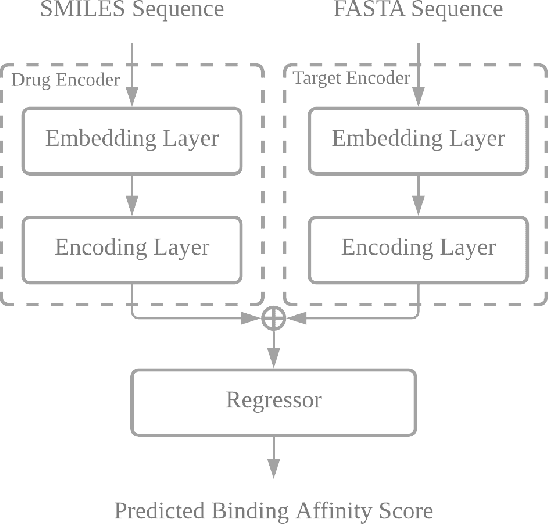
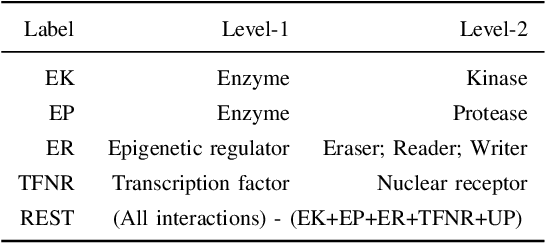
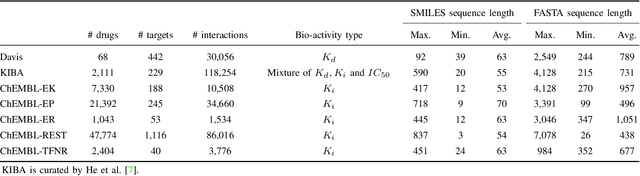
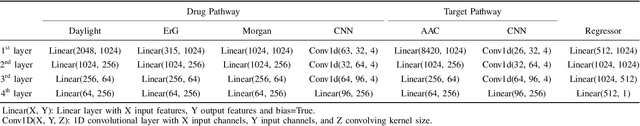
Abstract:Drug-target interaction (DTI) prediction plays a crucial role in drug discovery, and deep learning approaches have achieved state-of-the-art performance in this field. We introduce an ensemble of deep learning models (EnsembleDLM) for DTI prediction. EnsembleDLM only uses the sequence information of chemical compounds and proteins, and it aggregates the predictions from multiple deep neural networks. This approach not only achieves state-of-the-art performance in Davis and KIBA datasets but also reaches cutting-edge performance in the cross-domain applications across different bio-activity types and different protein classes. We also demonstrate that EnsembleDLM achieves a good performance (Pearson correlation coefficient and concordance index > 0.8) in the new domain with approximately 50% transfer learning data, i.e., the training set has twice as much data as the test set.
Predicting Clinical Outcome of Stroke Patients with Tractographic Feature
Sep 03, 2019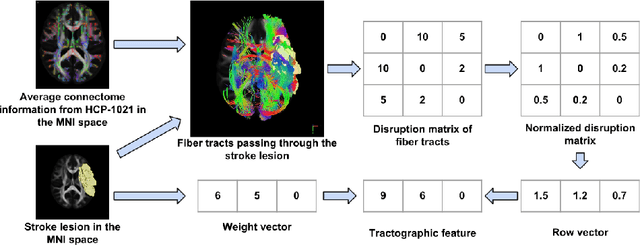
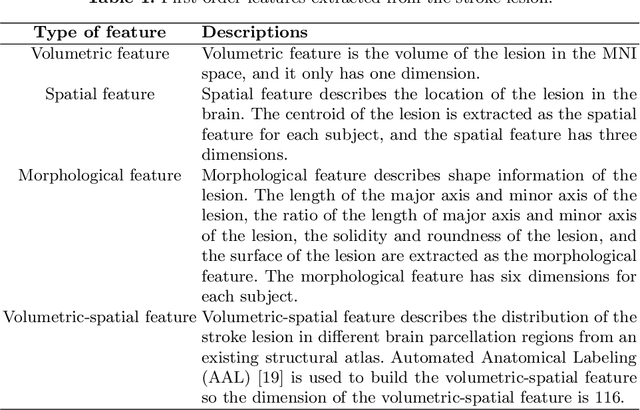
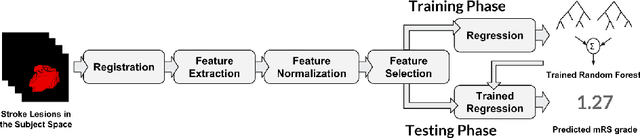
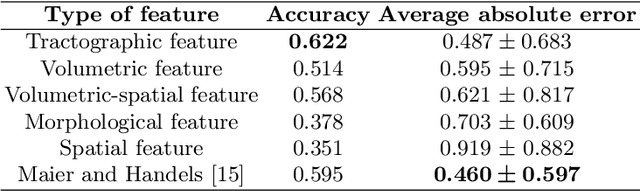
Abstract:The volume of stroke lesion is the gold standard for predicting the clinical outcome of stroke patients. However, the presence of stroke lesion may cause neural disruptions to other brain regions, and these potentially damaged regions may affect the clinical outcome of stroke patients. In this paper, we introduce the tractographic feature to capture these potentially damaged regions and predict the modified Rankin Scale (mRS), which is a widely used outcome measure in stroke clinical trials. The tractographic feature is built from the stroke lesion and average connectome information from a group of normal subjects. The tractographic feature takes into account different functional regions that may be affected by the stroke, thus complementing the commonly used stroke volume features. The proposed tractographic feature is tested on a public stroke benchmark Ischemic Stroke Lesion Segmentation 2017 and achieves higher accuracy than the stroke volume and the state-of-the-art feature on predicting the mRS grades of stroke patients. In addition, the tractographic feature also yields a lower average absolute error than the commonly used stroke volume feature.
Improving 3D U-Net for Brain Tumor Segmentation by Utilizing Lesion Prior
Jun 29, 2019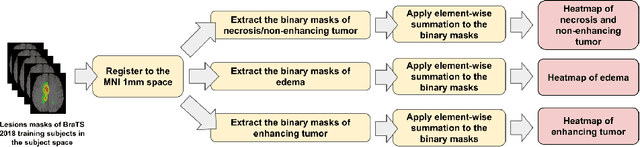
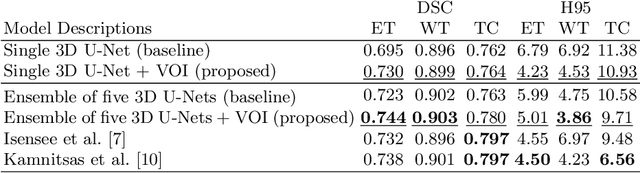
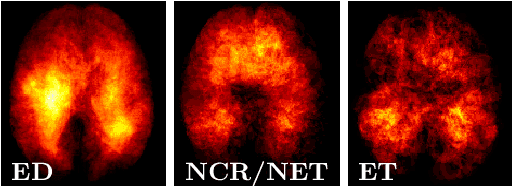

Abstract:We propose a novel, simple and effective method to integrate lesion prior and a 3D U-Net for improving brain tumor segmentation. First, we utilize the ground-truth brain tumor lesions from a group of patients to generate the heatmaps of different types of lesions. These heatmaps are used to create the volume-of-interest (VOI) map which contains prior information about brain tumor lesions. The VOI map is then integrated with the multimodal MR images and input to a 3D U-Net for segmentation. The proposed method is evaluated on a public benchmark dataset, and the experimental results show that the proposed feature fusion method achieves an improvement over the baseline methods. In addition, our proposed method also achieves a competitive performance compared to state-of-the-art methods.
Predicting Fluid Intelligence of Children using T1-weighted MR Images and a StackNet
May 07, 2019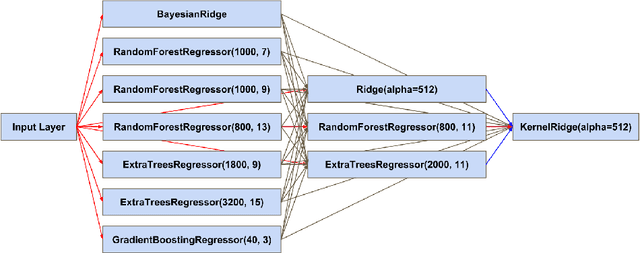

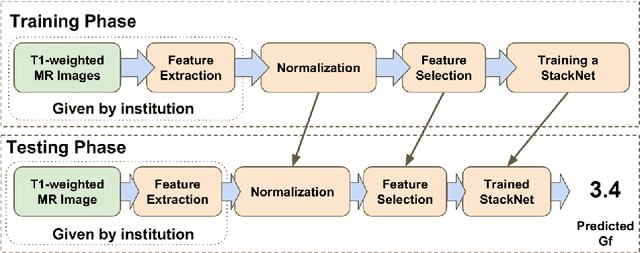
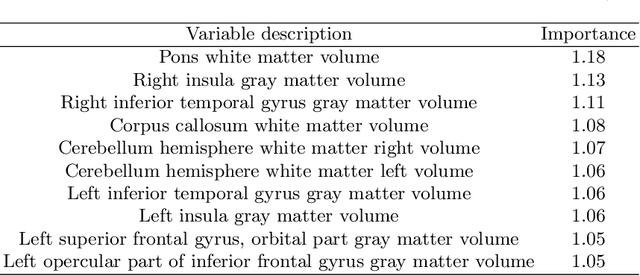
Abstract:In this work, we utilize T1-weighted MR images and StackNet to predict fluid intelligence in adolescents. Our framework includes feature extraction, feature normalization, feature denoising, feature selection, training a StackNet, and predicting fluid intelligence. The extracted feature is the distribution of different brain tissues in different brain parcellation regions. The proposed StackNet consists of three layers and 11 models. Each layer uses the predictions from all previous layers including the input layer. The proposed StackNet is tested on a public benchmark Adolescent Brain Cognitive Development Neurocognitive Prediction Challenge 2019 and achieves a mean squared error of 82.42 on the combined training and validation set with 10-fold cross-validation. In addition, the proposed StackNet also achieves a mean squared error of 94.25 on the testing data. The source code is available on GitHub.
Accurate 3D Cell Segmentation using Deep Feature and CRF Refinement
Feb 13, 2019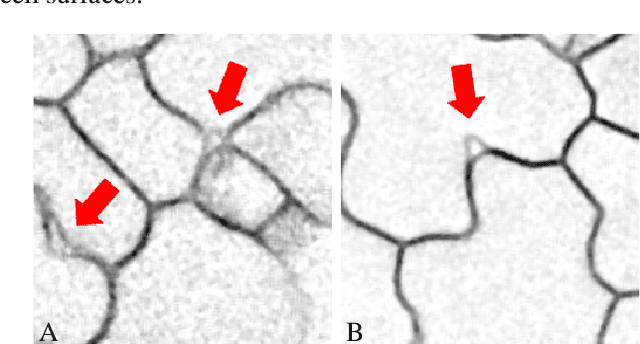
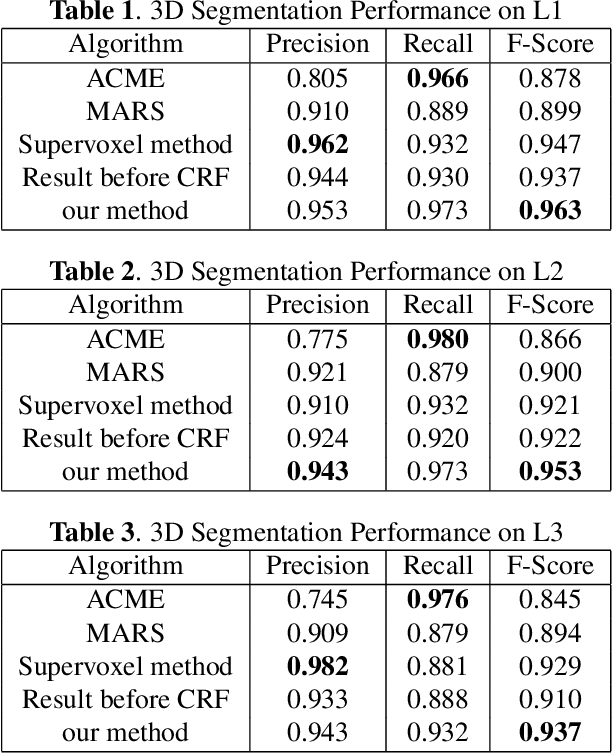

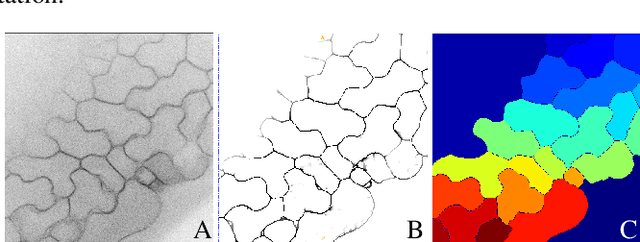
Abstract:We consider the problem of accurately identifying cell boundaries and labeling individual cells in confocal microscopy images, specifically, 3D image stacks of cells with tagged cell membranes. Precise identification of cell boundaries, their shapes, and quantifying inter-cellular space leads to a better understanding of cell morphogenesis. Towards this, we outline a cell segmentation method that uses a deep neural network architecture to extract a confidence map of cell boundaries, followed by a 3D watershed algorithm and a final refinement using a conditional random field. In addition to improving the accuracy of segmentation compared to other state-of-the-art methods, the proposed approach also generalizes well to different datasets without the need to retrain the network for each dataset. Detailed experimental results are provided, and the source code is available on GitHub.
Fully Automated Volumetric Classification in CT Scans for Diagnosis and Analysis of Normal Pressure Hydrocephalus
Jan 25, 2019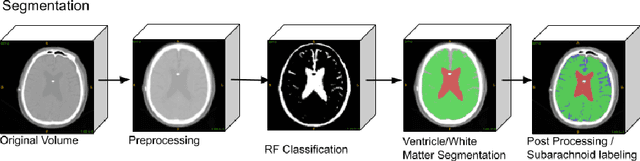
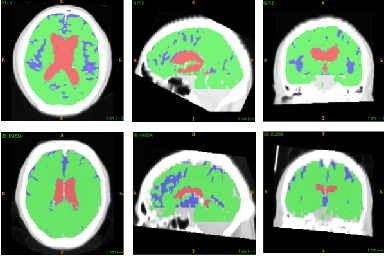
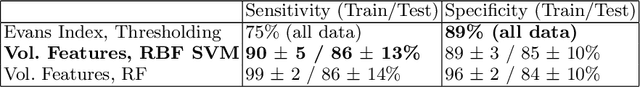
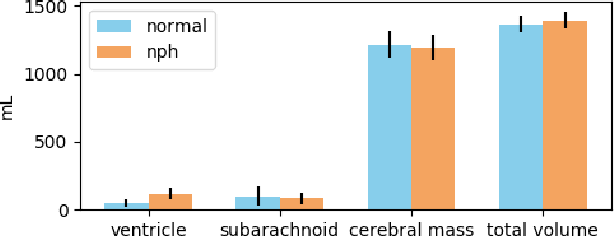
Abstract:Normal Pressure Hydrocephalus (NPH) is one of the few reversible forms of dementia. Due to their low cost and versatility, Computed Tomography (CT) scans have long been used as an aid to help diagnose intracerebral anomalies such as NPH. However, because CT imaging presents 2-dimensional slices of a 3-dimensional volume, recapitulating the ventricular space in 3-dimensions to facilitate the diagnosis of NPH poses numerous challenges such as head rotation and human error. As such, no well-defined and effective protocol currently exists for the analysis of CT scan-based ventricular, white matter and subarachnoid space volumes in the setting of NPH. The Evan's ratio, an approximation of the ratio of ventricle to brain volume using only one 2D slice of the scan, has been proposed but is not robust. Instead of manually measuring a 2-dimensional proxy for the ratio of ventricle volume to brain volume, this study proposes an automated method of calculating the brain volumes for better recognition of NPH from a radiological standpoint. The method first aligns the subject CT volume to a common space through an affine transformation, then uses a random forest classifier to mask relevant tissue types. A 3D morphological segmentation method is used to partition the brain volume, which in turn is used to train machine learning methods to classify the subjects into non-NPH vs. NPH based on volumetric information.
Brain Tumor Segmentation and Tractographic Feature Extraction from Structural MR Images for Overall Survival Prediction
Oct 10, 2018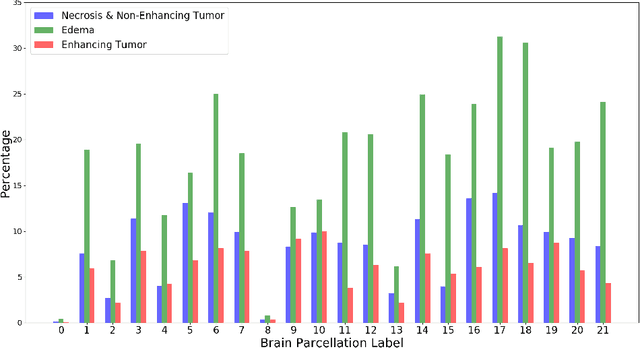
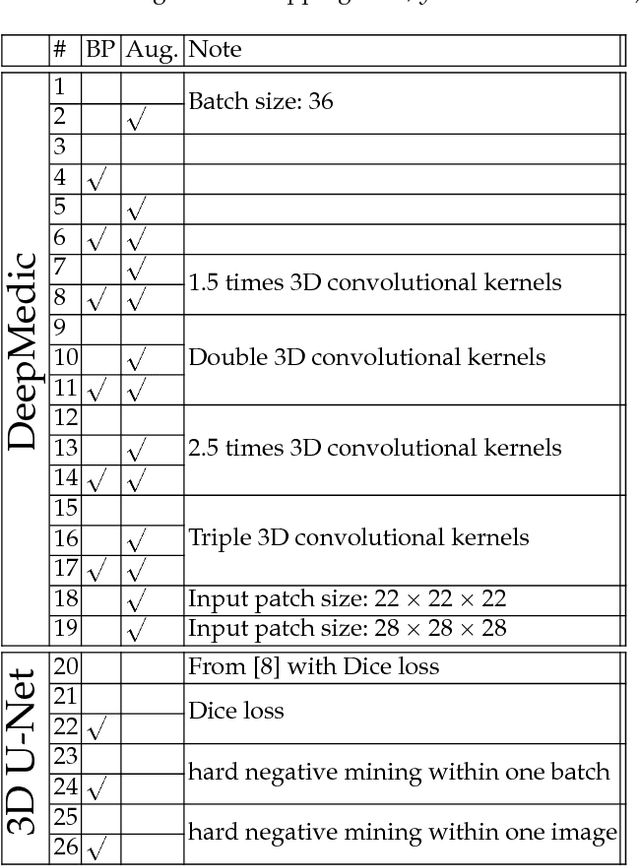
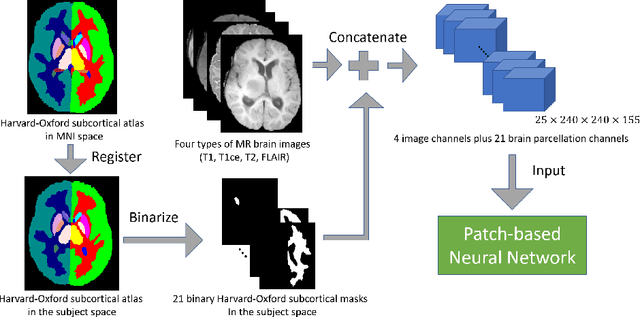

Abstract:This paper introduces a novel methodology to integrate human brain connectomics and parcellation for brain tumor segmentation and survival prediction. For segmentation, we utilize an existing brain parcellation atlas in the MNI152 1mm space and map this parcellation to each individual subject data. We use deep neural network architectures together with hard negative mining to achieve the final voxel level classification. For survival prediction, we present a new method for combining features from connectomics data, brain parcellation information, and the brain tumor mask. We leverage the average connectome information from the Human Connectome Project and map each subject brain volume onto this common connectome space. From this, we compute tractographic features that describe potential neural disruptions due to the brain tumor. These features are then used to predict the overall survival of the subjects. The main novelty in the proposed methods is the use of normalized brain parcellation data and tractography data from the human connectome project for analyzing MR images for segmentation and survival prediction. Experimental results are reported on the BraTS2018 data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge