Daniel B. Szymanski
Deep Learning Enabled Time-Lapse 3D Cell Analysis
Aug 17, 2022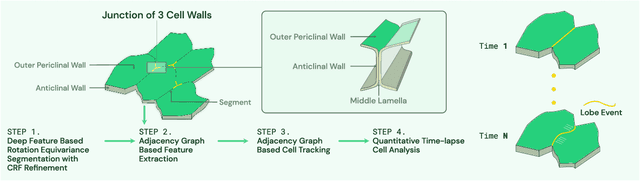
Abstract:This paper presents a method for time-lapse 3D cell analysis. Specifically, we consider the problem of accurately localizing and quantitatively analyzing sub-cellular features, and for tracking individual cells from time-lapse 3D confocal cell image stacks. The heterogeneity of cells and the volume of multi-dimensional images presents a major challenge for fully automated analysis of morphogenesis and development of cells. This paper is motivated by the pavement cell growth process, and building a quantitative morphogenesis model. We propose a deep feature based segmentation method to accurately detect and label each cell region. An adjacency graph based method is used to extract sub-cellular features of the segmented cells. Finally, the robust graph based tracking algorithm using multiple cell features is proposed for associating cells at different time instances. Extensive experiment results are provided and demonstrate the robustness of the proposed method. The code is available on Github and the method is available as a service through the BisQue portal.
Accurate 3D Cell Segmentation using Deep Feature and CRF Refinement
Feb 13, 2019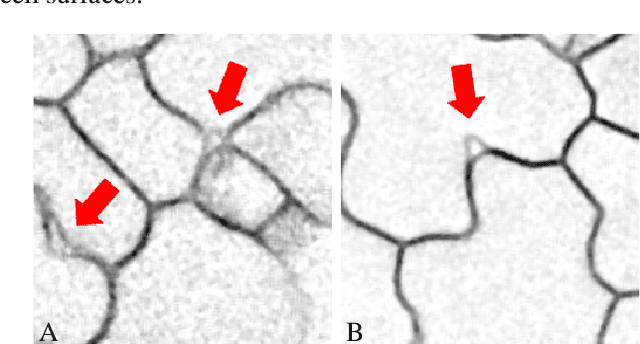
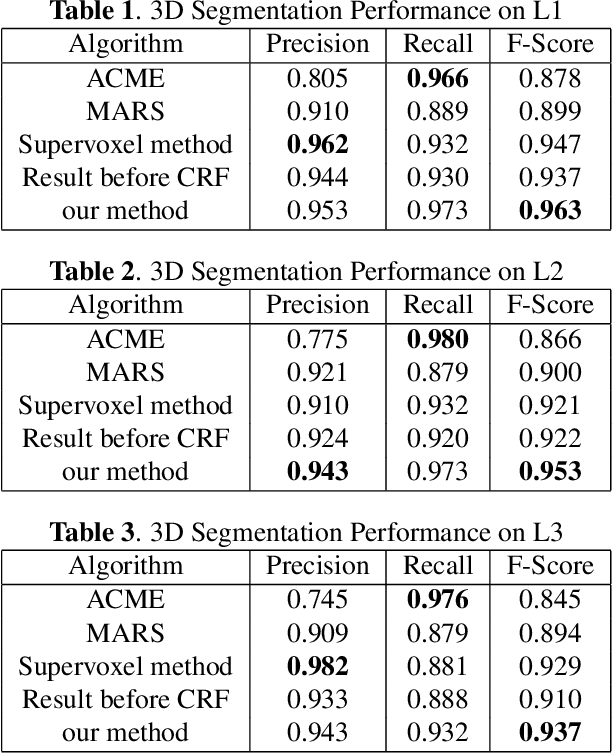

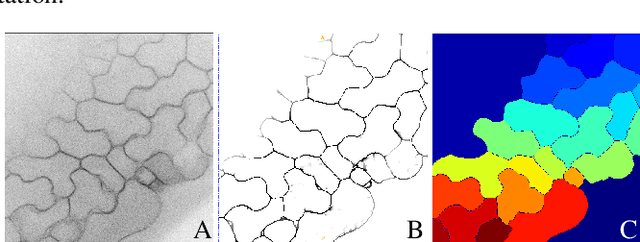
Abstract:We consider the problem of accurately identifying cell boundaries and labeling individual cells in confocal microscopy images, specifically, 3D image stacks of cells with tagged cell membranes. Precise identification of cell boundaries, their shapes, and quantifying inter-cellular space leads to a better understanding of cell morphogenesis. Towards this, we outline a cell segmentation method that uses a deep neural network architecture to extract a confidence map of cell boundaries, followed by a 3D watershed algorithm and a final refinement using a conditional random field. In addition to improving the accuracy of segmentation compared to other state-of-the-art methods, the proposed approach also generalizes well to different datasets without the need to retrain the network for each dataset. Detailed experimental results are provided, and the source code is available on GitHub.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge