Pierre Boulanger
Accelerated 3D-3D rigid registration of echocardiographic images obtained from apical window using particle filter
Apr 28, 2025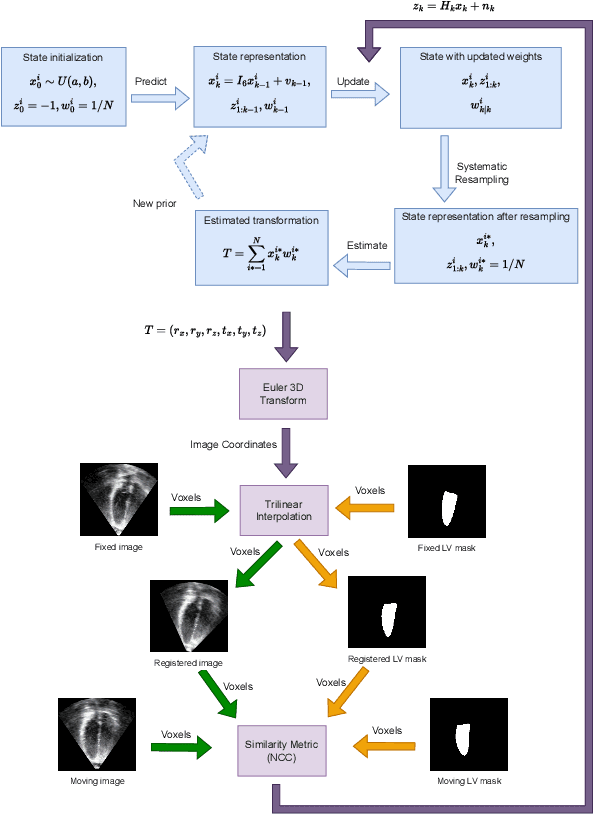



Abstract:The perfect alignment of 3D echocardiographic images captured from various angles has improved image quality and broadened the field of view. This study proposes an accelerated sequential Monte Carlo (SMC) algorithm for 3D-3D rigid registration of transthoracic echocardiographic images with significant and limited overlap taken from apical window that is robust to the noise and intensity variation in ultrasound images. The algorithm estimates the translational and rotational components of the rigid transform through an iterative process and requires an initial approximation of the rotation and translation limits. We perform registration in two ways: the image-based registration computes the transform to align the end-diastolic frame of the apical nonstandard image to the apical standard image and applies the same transform to all frames of the cardiac cycle, whereas the mask-based registration approach uses the binary masks of the left ventricle in the same way. The SMC and exhaustive search (EX) algorithms were evaluated for 4D temporal sequences recorded from 7 volunteers who participated in a study conducted at the Mazankowski Alberta Heart Institute. The evaluations demonstrate that the mask-based approach of the accelerated SMC yielded a Dice score value of 0.819 +/- 0.045 for the left ventricle and gained 16.7x speedup compared to the CPU version of the SMC algorithm.
Personalizing Exposure Therapy via Reinforcement Learning
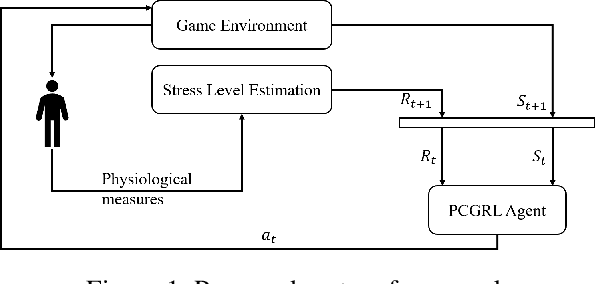
Apr 18, 2025Abstract:Personalized therapy, in which a therapeutic practice is adapted to an individual patient, can lead to improved health outcomes. Typically, this is accomplished by relying on a therapist's training and intuition along with feedback from a patient. However, this requires the therapist to become an expert on any technological components, such as in the case of Virtual Reality Exposure Therapy (VRET). While there exist approaches to automatically adapt therapeutic content to a patient, they generally rely on hand-authored, pre-defined rules, which may not generalize to all individuals. In this paper, we propose an approach to automatically adapt therapeutic content to patients based on physiological measures. We implement our approach in the context of virtual reality arachnophobia exposure therapy, and rely on experience-driven procedural content generation via reinforcement learning (EDPCGRL) to generate virtual spiders to match an individual patient. Through a human subject study, we demonstrate that our system significantly outperforms a more common rules-based method, highlighting its potential for enhancing personalized therapeutic interventions.
Stress Detection from Photoplethysmography in a Virtual Reality Environment
Sep 25, 2024Abstract:Personalized virtual reality exposure therapy is a therapeutic practice that can adapt to an individual patient, leading to better health outcomes. Measuring a patient's mental state to adjust the therapy is a critical but difficult task. Most published studies use subjective methods to estimate a patient's mental state, which can be inaccurate. This article proposes a virtual reality exposure therapy (VRET) platform capable of assessing a patient's mental state using non-intrusive and widely available physiological signals such as photoplethysmography (PPG). In a case study, we evaluate how PPG signals can be used to detect two binary classifications: peaceful and stressful states. Sixteen healthy subjects were exposed to the two VR environments (relaxed and stressful). Using LOSO cross-validation, our best classification model could predict the two states with a 70.6% accuracy which outperforms many more complex approaches.
Spiders Based on Anxiety: How Reinforcement Learning Can Deliver Desired User Experience in Virtual Reality Personalized Arachnophobia Treatment
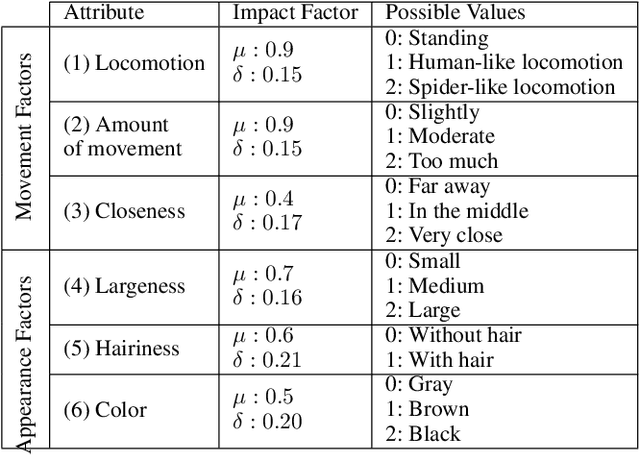
Sep 25, 2024Abstract:The need to generate a spider to provoke a desired anxiety response arises in the context of personalized virtual reality exposure therapy (VRET), a treatment approach for arachnophobia. This treatment involves patients observing virtual spiders in order to become desensitized and decrease their phobia, which requires that the spiders elicit specific anxiety responses. However, VRET approaches tend to require therapists to hand-select the appropriate spider for each patient, which is a time-consuming process and takes significant technical knowledge and patient insight. While automated methods exist, they tend to employ rules-based approaches with minimal ability to adapt to specific users. To address these challenges, we present a framework for VRET utilizing procedural content generation (PCG) and reinforcement learning (RL), which automatically adapts a spider to elicit a desired anxiety response. We demonstrate the superior performance of this system compared to a more common rules-based VRET method.
Grey-box Bayesian Optimization for Sensor Placement in Assisted Living Environments
Sep 11, 2023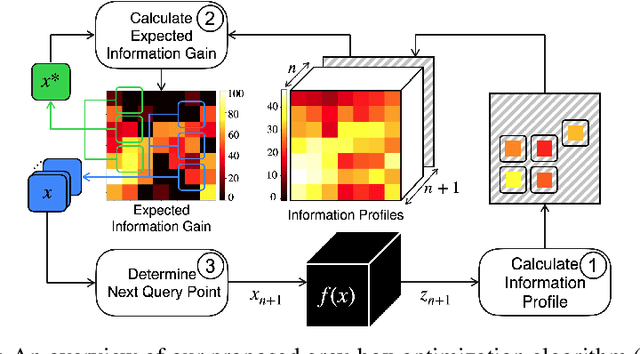
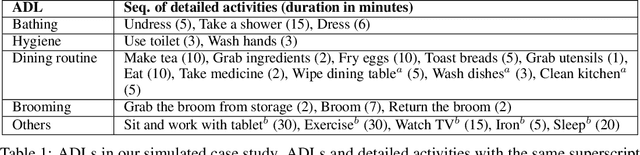
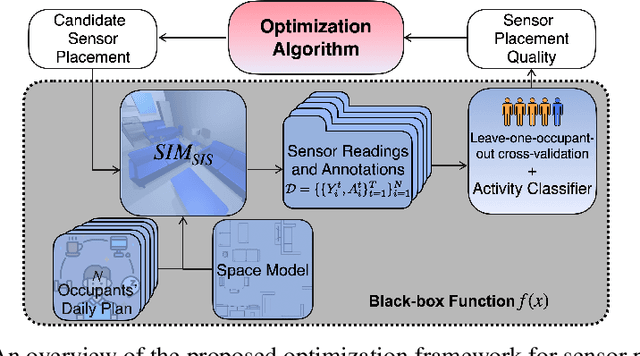
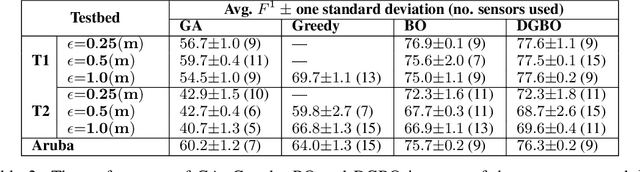
Abstract:Optimizing the configuration and placement of sensors is crucial for reliable fall detection, indoor localization, and activity recognition in assisted living spaces. We propose a novel, sample-efficient approach to find a high-quality sensor placement in an arbitrary indoor space based on grey-box Bayesian optimization and simulation-based evaluation. Our key technical contribution lies in capturing domain-specific knowledge about the spatial distribution of activities and incorporating it into the iterative selection of query points in Bayesian optimization. Considering two simulated indoor environments and a real-world dataset containing human activities and sensor triggers, we show that our proposed method performs better compared to state-of-the-art black-box optimization techniques in identifying high-quality sensor placements, leading to accurate activity recognition in terms of F1-score, while also requiring a significantly lower (51.3% on average) number of expensive function queries.
Ischemic Stroke Lesion Prediction using imbalanced Temporal Deep Gaussian Process (iTDGP)
Nov 16, 2022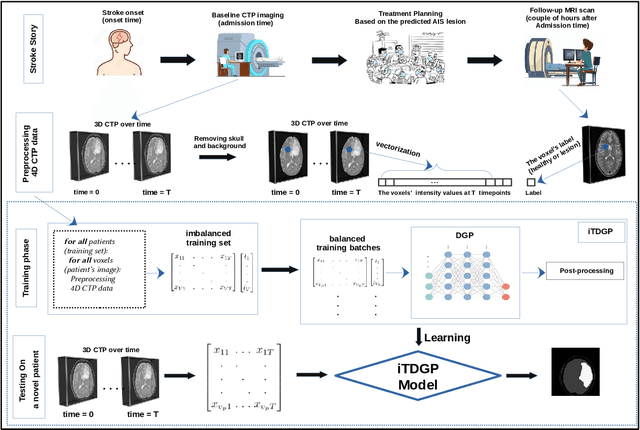
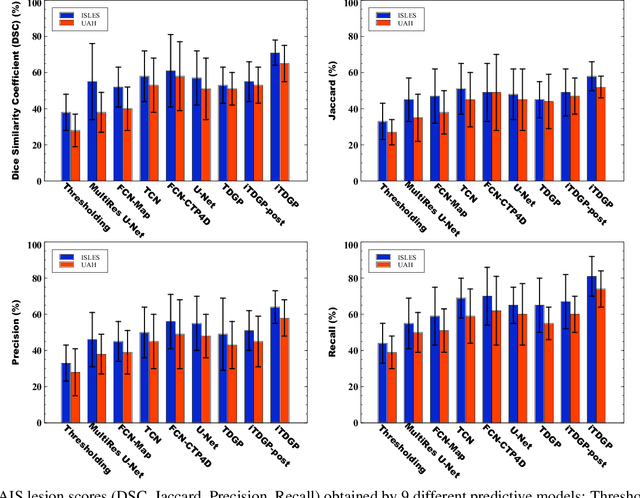
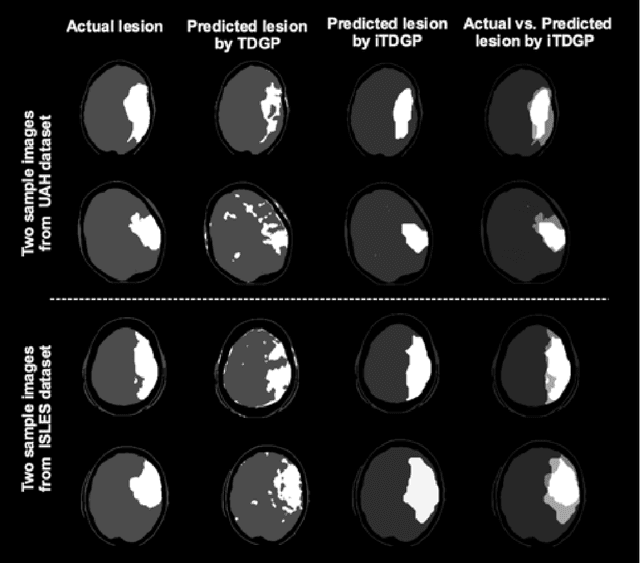
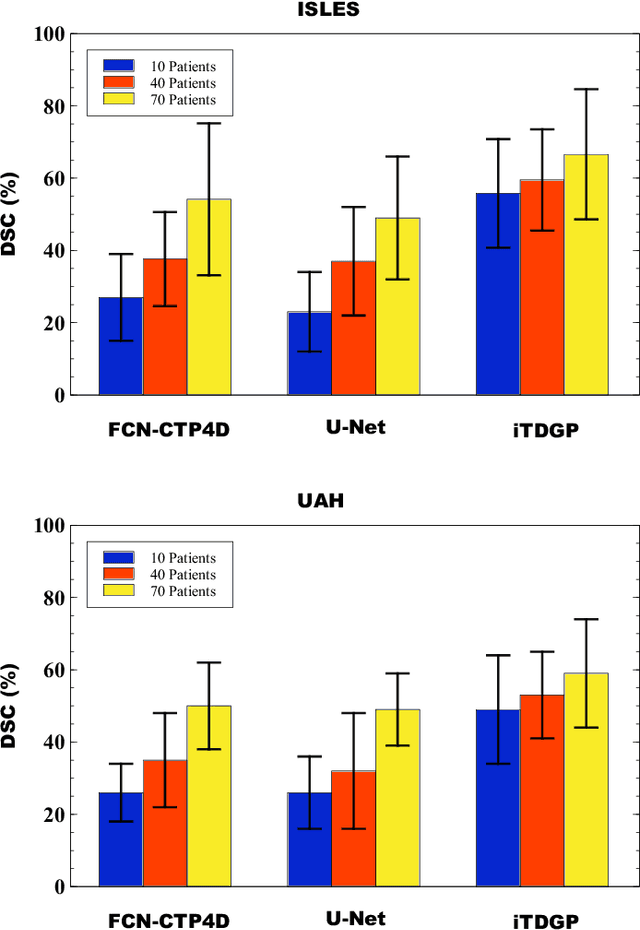
Abstract:As one of the leading causes of mortality and disability worldwide, Acute Ischemic Stroke (AIS) occurs when the blood supply to the brain is suddenly interrupted because of a blocked artery. Within seconds of AIS onset, the brain cells surrounding the blocked artery die, which leads to the progression of the lesion. The automated and precise prediction of the existing lesion plays a vital role in the AIS treatment planning and prevention of further injuries. The current standard AIS assessment method, which thresholds the 3D measurement maps extracted from Computed Tomography Perfusion (CTP) images, is not accurate enough. Due to this fact, in this article, we propose the imbalanced Temporal Deep Gaussian Process (iTDGP), a probabilistic model that can improve AIS lesions prediction by using baseline CTP time series. Our proposed model can effectively extract temporal information from the CTP time series and map it to the class labels of the brain's voxels. In addition, by using batch training and voxel-level analysis iTDGP can learn from a few patients and it is robust against imbalanced classes. Moreover, our model incorporates a post-processor capable of improving prediction accuracy using spatial information. Our comprehensive experiments, on the ISLES 2018 and the University of Alberta Hospital (UAH) datasets, show that iTDGP performs better than state-of-the-art AIS lesion predictors, obtaining the (cross-validation) Dice score of 71.42% and 65.37% with a significant p<0.05, respectively.
Arachnophobia Exposure Therapy using Experience-driven Procedural Content Generation via Reinforcement Learning (EDPCGRL)
Oct 07, 2021

Abstract:Personalized therapy, in which a therapeutic practice is adapted to an individual patient, leads to better health outcomes. Typically, this is accomplished by relying on a therapist's training and intuition along with feedback from a patient. While there exist approaches to automatically adapt therapeutic content to a patient, they rely on hand-authored, pre-defined rules, which may not generalize to all individuals. In this paper, we propose an approach to automatically adapt therapeutic content to patients based on physiological measures. We implement our approach in the context of arachnophobia exposure therapy, and rely on experience-driven procedural content generation via reinforcement learning (EDPCGRL) to generate virtual spiders to match an individual patient. In this initial implementation, and due to the ongoing pandemic, we make use of virtual or artificial humans implemented based on prior arachnophobia psychology research. Our EDPCGRL method is able to more quickly adapt to these virtual humans with high accuracy in comparison to existing, search-based EDPCG approaches.
* 8 pages, 3 figures, AIIDE 2021 Poster
A New Semi-Automated Algorithm for Volumetric Segmentation of the Left Ventricle in Temporal 3D Echocardiography Sequences
Sep 03, 2021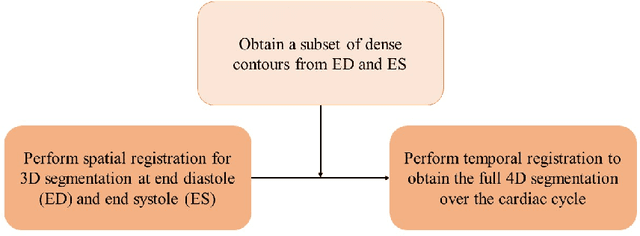
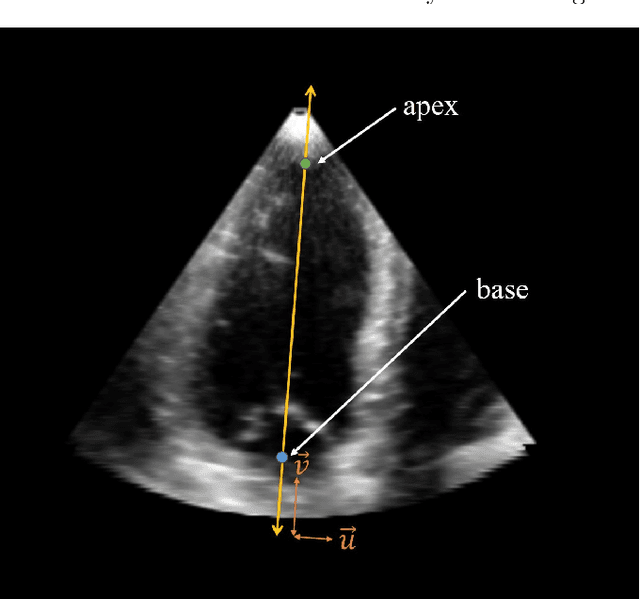
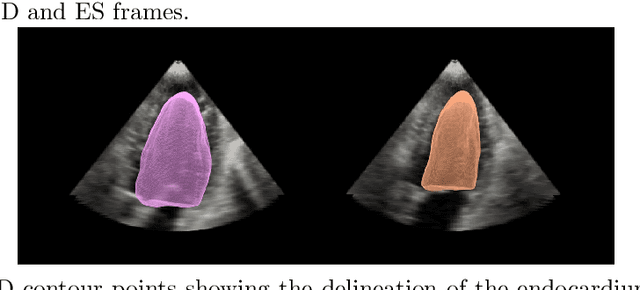
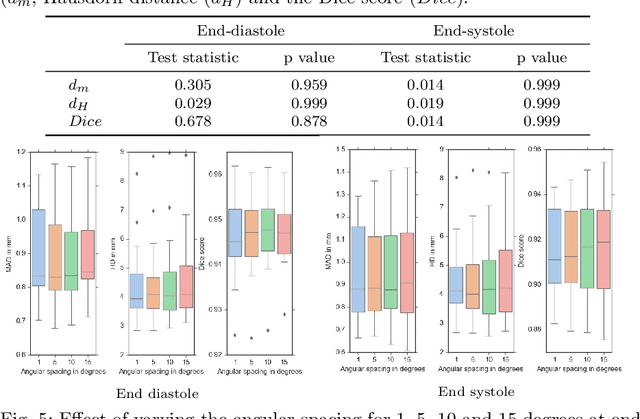
Abstract:Purpose: Echocardiography is commonly used as a non-invasive imaging tool in clinical practice for the assessment of cardiac function. However, delineation of the left ventricle is challenging due to the inherent properties of ultrasound imaging, such as the presence of speckle noise and the low signal-to-noise ratio. Methods: We propose a semi-automated segmentation algorithm for the delineation of the left ventricle in temporal 3D echocardiography sequences. The method requires minimal user interaction and relies on a diffeomorphic registration approach. Advantages of the method include no dependence on prior geometrical information, training data, or registration from an atlas. Results: The method was evaluated using three-dimensional ultrasound scan sequences from 18 patients from the Mazankowski Alberta Heart Institute, Edmonton, Canada, and compared to manual delineations provided by an expert cardiologist and four other registration algorithms. The segmentation approach yielded the following results over the cardiac cycle: a mean absolute difference of 1.01 (0.21) mm, a Hausdorff distance of 4.41 (1.43) mm, and a Dice overlap score of 0.93 (0.02). Conclusions: The method performed well compared to the four other registration algorithms.
* 22 pages, 8 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge