Peidi Xu
Locally orderless networks
Jun 19, 2024Abstract:We present Locally Orderless Networks (LON) and its theoretic foundation which links it to Convolutional Neural Networks (CNN), to Scale-space histograms, and measurement theory. The key elements are a regular sampling of the bias and the derivative of the activation function. We compare LON, CNN, and Scale-space histograms on prototypical single-layer networks. We show how LON and CNN can emulate each other, how LON expands the set of functionals computable to non-linear functions such as squaring. We demonstrate simple networks which illustrate the improved performance of LON over CNN on simple tasks for estimating the gradient magnitude squared, for regressing shape area and perimeter lengths, and for explainability of individual pixels' influence on the result.
Extremely weakly-supervised blood vessel segmentation with physiologically based synthesis and domain adaptation
May 26, 2023



Abstract:Accurate analysis and modeling of renal functions require a precise segmentation of the renal blood vessels. Micro-CT scans provide image data at higher resolutions, making more small vessels near the renal cortex visible. Although deep-learning-based methods have shown state-of-the-art performance in automatic blood vessel segmentations, they require a large amount of labeled training data. However, voxel-wise labeling in micro-CT scans is extremely time-consuming given the huge volume sizes. To mitigate the problem, we simulate synthetic renal vascular trees physiologically while generating corresponding scans of the simulated trees by training a generative model on unlabeled scans. This enables the generative model to learn the mapping implicitly without the need for explicit functions to emulate the image acquisition process. We further propose an additional segmentation branch over the generative model trained on the generated scans. We demonstrate that the model can directly segment blood vessels on real scans and validate our method on both 3D micro-CT scans of rat kidneys and a proof-of-concept experiment on 2D retinal images. Code and 3D results are available at https://github.com/miccai2023anony/RenalVesselSeg
A Hybrid Approach to Full-Scale Reconstruction of Renal Arterial Network
Mar 03, 2023Abstract:The renal vasculature, acting as a resource distribution network, plays an important role in both the physiology and pathophysiology of the kidney. However, no imaging techniques allow an assessment of the structure and function of the renal vasculature due to limited spatial and temporal resolution. To develop realistic computer simulations of renal function, and to develop new image-based diagnostic methods based on artificial intelligence, it is necessary to have a realistic full-scale model of the renal vasculature. We propose a hybrid framework to build subject-specific models of the renal vascular network by using semi-automated segmentation of large arteries and estimation of cortex area from a micro-CT scan as a starting point, and by adopting the Global Constructive Optimization algorithm for generating smaller vessels. Our results show a statistical correspondence between the reconstructed data and existing anatomical data obtained from a rat kidney with respect to morphometric and hemodynamic parameters.
Auto-segmentation of Hip Joints using MultiPlanar UNet with Transfer learning
Aug 18, 2022
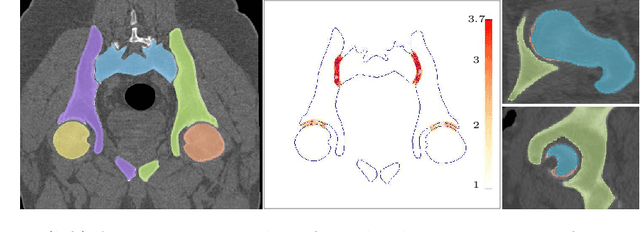


Abstract:Accurate geometry representation is essential in developing finite element models. Although generally good, deep-learning segmentation approaches with only few data have difficulties in accurately segmenting fine features, e.g., gaps and thin structures. Subsequently, segmented geometries need labor-intensive manual modifications to reach a quality where they can be used for simulation purposes. We propose a strategy that uses transfer learning to reuse datasets with poor segmentation combined with an interactive learning step where fine-tuning of the data results in anatomically accurate segmentations suitable for simulations. We use a modified MultiPlanar UNet that is pre-trained using inferior hip joint segmentation combined with a dedicated loss function to learn the gap regions and post-processing to correct tiny inaccuracies on symmetric classes due to rotational invariance. We demonstrate this robust yet conceptually simple approach applied with clinically validated results on publicly available computed tomography scans of hip joints. Code and resulting 3D models are available at: https://github.com/MICCAI2022-155/AuToSeg}
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge