Oren Solomon
Compressed Ultrasound Imaging:from Sub-Nyquist Rates to Super-Resolution
Jul 23, 2021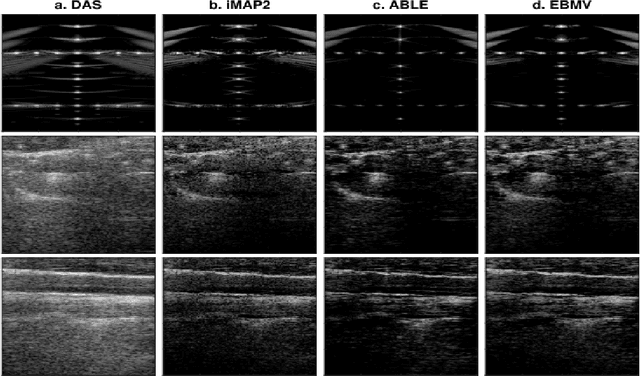
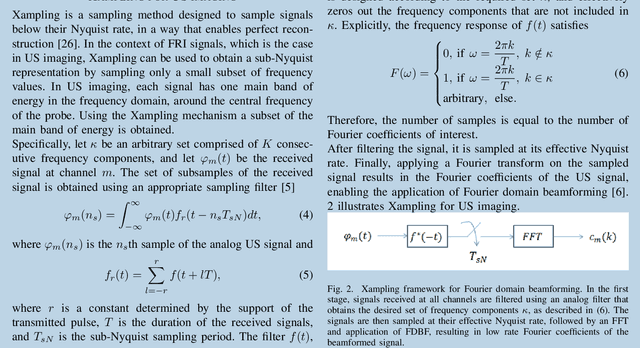

Abstract:The multi-billion dollar, worldwide medical ultrasound (US) market continues to grow annually. Its non-ionizing nature, real-time capabilities and relatively low cost, compared to other imaging modalities, have led to significant applications in many different fields, including cardiology, angiology, obstetrics and emergency medicine. Facilitated by ongoing innovations, US continues to change rules and norms regarding patient screening, diagnosis and surgery. This huge and promising market is constantly driven by new imaging and processing techniques. From 3D images to sophisticated software, hardware and portability improvements, it is clear that the status of US as one of the leading medical imaging technologies is ensured for many years ahead. However, as imaging systems evolve, new engineering challenges emerge. Acquisition, transmission and processing of huge amounts of data are common for all ultrasound-based imaging modalities. Moreover, achieving higher resolution is constantly on demand, as improved diagnosis could be achieved by better visualization of organs and blood vessels deep within tissues. In this article, our goal is to motivate further interest and research in emerging processing techniques, as well as their applications in medical ultrasound, enabled by recent advancements in signal processing algorithms and deep learning. We address some of the primary challenges and potential remedies from a signal processing perspective, by exploiting the inherent structure of the received US signal.
Deep Cerebellar Nuclei Segmentation via Semi-Supervised Deep Context-Aware Learning from 7T Diffusion MRI
May 12, 2020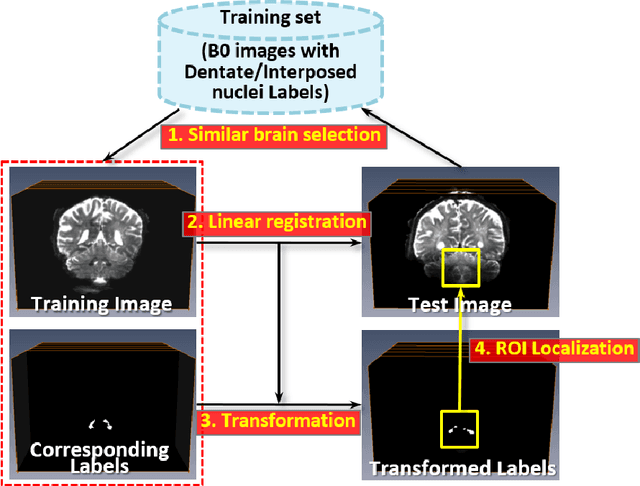

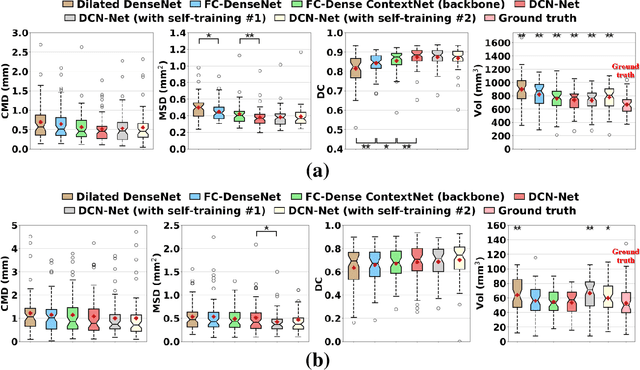
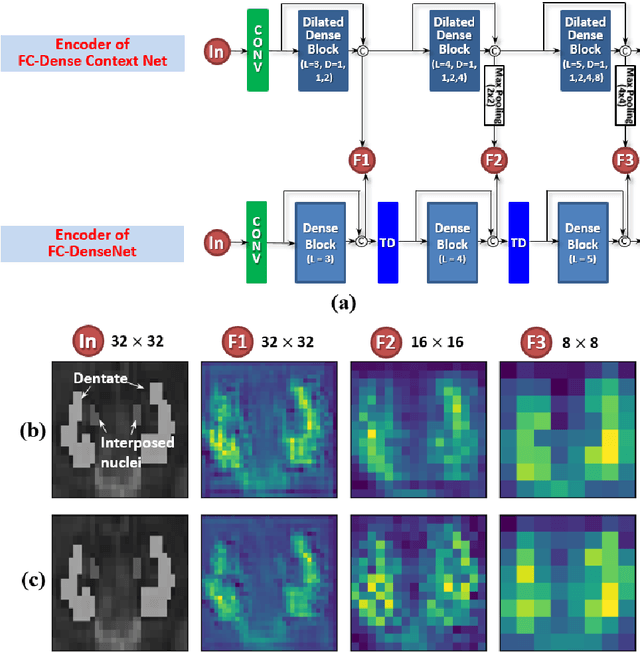
Abstract:Deep cerebellar nuclei are a key structure of the cerebellum that are involved in processing motor and sensory information. It is thus a crucial step to accurately segment deep cerebellar nuclei for the understanding of the cerebellum system and its utility in deep brain stimulation treatment. However, it is challenging to clearly visualize such small nuclei under standard clinical magnetic resonance imaging (MRI) protocols and therefore precise segmentation is not feasible. Recent advances in 7 Tesla (T) MRI technology and great potential of deep neural networks facilitate automatic patient-specific segmentation. In this paper, we propose a novel deep learning framework (referred to as DCN-Net) for fast, accurate, and robust patient-specific segmentation of deep cerebellar dentate and interposed nuclei on 7T diffusion MRI. DCN-Net effectively encodes contextual information on the patch images without consecutive pooling operations and adding complexity via proposed dilated dense blocks. During the end-to-end training, label probabilities of dentate and interposed nuclei are independently learned with a hybrid loss, handling highly imbalanced data. Finally, we utilize self-training strategies to cope with the problem of limited labeled data. To this end, auxiliary dentate and interposed nuclei labels are created on unlabeled data by using DCN-Net trained on manual labels. We validate the proposed framework using 7T B0 MRIs from 60 subjects. Experimental results demonstrate that DCN-Net provides better segmentation than atlas-based deep cerebellar nuclei segmentation tools and other state-of-the-art deep neural networks in terms of accuracy and consistency. We further prove the effectiveness of the proposed components within DCN-Net in dentate and interposed nuclei segmentation.
Deep Unfolded Robust PCA with Application to Clutter Suppression in Ultrasound
Nov 20, 2018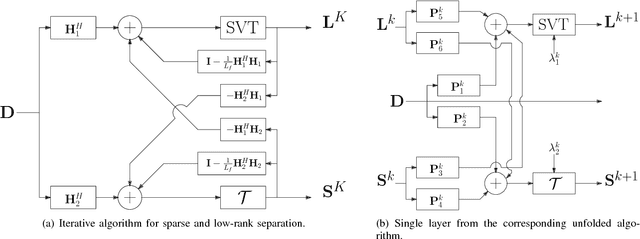
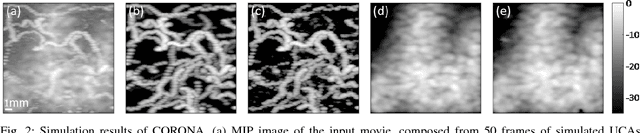
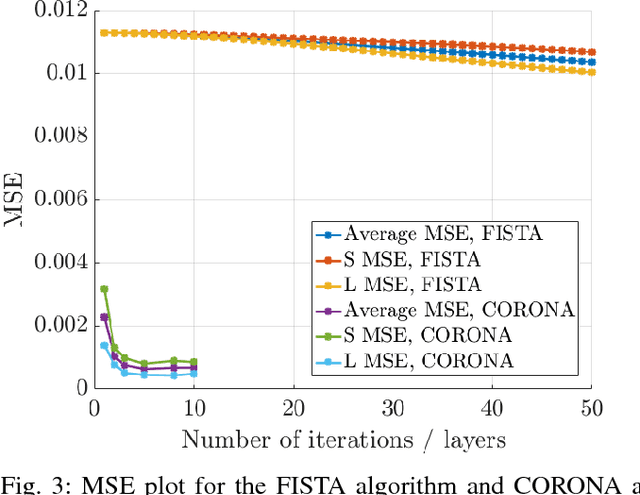
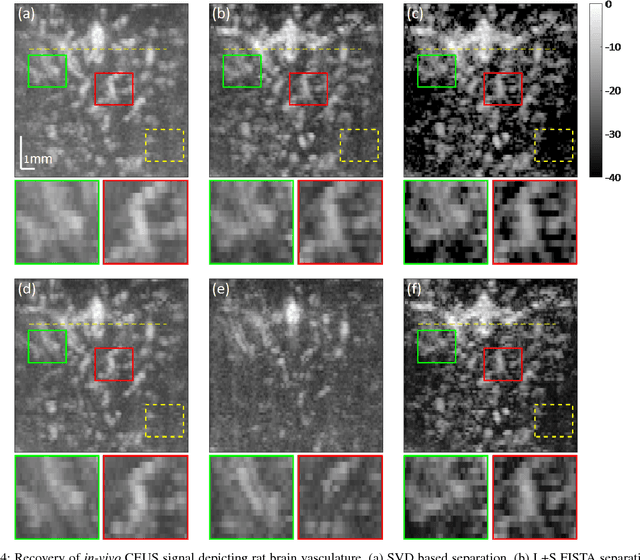
Abstract:Contrast enhanced ultrasound is a radiation-free imaging modality which uses encapsulated gas microbubbles for improved visualization of the vascular bed deep within the tissue. It has recently been used to enable imaging with unprecedented subwavelength spatial resolution by relying on super-resolution techniques. A typical preprocessing step in super-resolution ultrasound is to separate the microbubble signal from the cluttering tissue signal. This step has a crucial impact on the final image quality. Here, we propose a new approach to clutter removal based on robust principle component analysis (PCA) and deep learning. We begin by modeling the acquired contrast enhanced ultrasound signal as a combination of a low rank and sparse components. This model is used in robust PCA and was previously suggested in the context of ultrasound Doppler processing and dynamic magnetic resonance imaging. We then illustrate that an iterative algorithm based on this model exhibits improved separation of microbubble signal from the tissue signal over commonly practiced methods. Next, we apply the concept of deep unfolding to suggest a deep network architecture tailored to our clutter filtering problem which exhibits improved convergence speed and accuracy with respect to its iterative counterpart. We compare the performance of the suggested deep network on both simulations and in-vivo rat brain scans, with a commonly practiced deep-network architecture and the fast iterative shrinkage algorithm, and show that our architecture exhibits better image quality and contrast.
Super-resolution Ultrasound Localization Microscopy through Deep Learning
Apr 20, 2018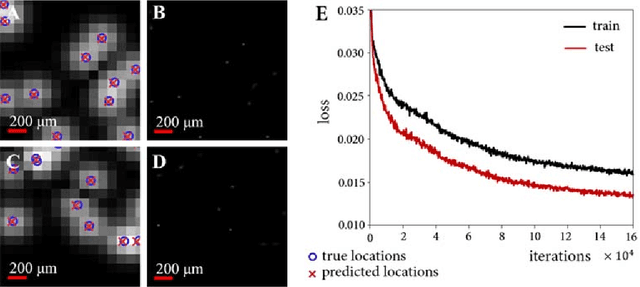
Abstract:Ultrasound localization microscopy has enabled super-resolution vascular imaging in laboratory environments through precise localization of individual ultrasound contrast agents across numerous imaging frames. However, analysis of high-density regions with significant overlaps among the agents' point spread responses yields high localization errors, constraining the technique to low-concentration conditions. As such, long acquisition times are required to sufficiently cover the vascular bed. In this work, we present a fast and precise method for obtaining super-resolution vascular images from high-density contrast-enhanced ultrasound imaging data. This method, which we term Deep Ultrasound Localization Microscopy (Deep-ULM), exploits modern deep learning strategies and employs a convolutional neural network to perform localization microscopy in dense scenarios. This end-to-end fully convolutional neural network architecture is trained effectively using on-line synthesized data, enabling robust inference in-vivo under a wide variety of imaging conditions. We show that deep learning attains super-resolution with challenging contrast-agent concentrations (microbubble densities), both in-silico as well as in-vivo, as we go from ultrasound scans of a rodent spinal cord in an experimental setting to standard clinically-acquired recordings in a human prostate. Deep-ULM achieves high quality sub-diffraction recovery, and is suitable for real-time applications, resolving about 135 high-resolution 64x64-patches per second on a standard PC. Exploiting GPU computation, this number increases to 2500 patches per second.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge