Oliwia Witczak
VISEM-Tracking: Human Spermatozoa Tracking Dataset
Dec 23, 2022Abstract:A manual assessment of sperm motility requires microscopy observation, which is challenging due to the fast-moving spermatozoa in the field of view. To obtain correct results, manual evaluation requires extensive training. Therefore, computer-assisted sperm analysis (CASA) has become increasingly used in clinics. Despite this, more data is needed to train supervised machine learning approaches in order to improve accuracy and reliability in the assessment of sperm motility and kinematics. In this regard, we provide a dataset called VISEM-Tracking with 20 video recordings of 30 seconds of wet sperm preparations with manually annotated bounding-box coordinates and a set of sperm characteristics analyzed by experts in the domain. In addition to the annotated data, we provide unlabeled video clips for easy-to-use access and analysis of the data via methods such as self- or unsupervised learning. As part of this paper, we present baseline sperm detection performances using the YOLOv5 deep learning model trained on the VISEM-Tracking dataset. As a result, we show that the dataset can be used to train complex deep learning models to analyze spermatozoa. The dataset is publicly available at https://zenodo.org/record/7293726.
Machine Learning-Based Analysis of Sperm Videos and Participant Data for Male Fertility Prediction
Oct 29, 2019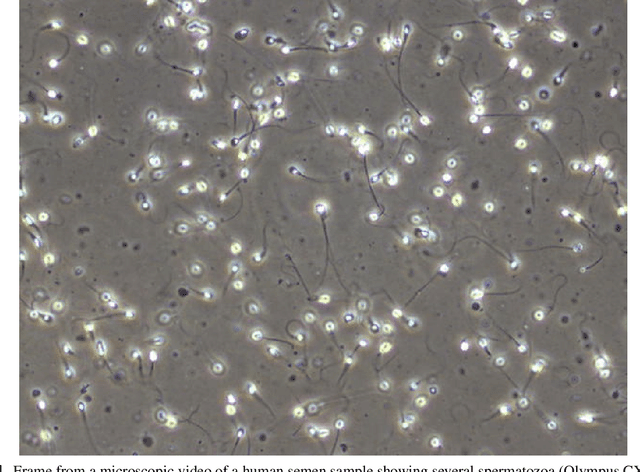
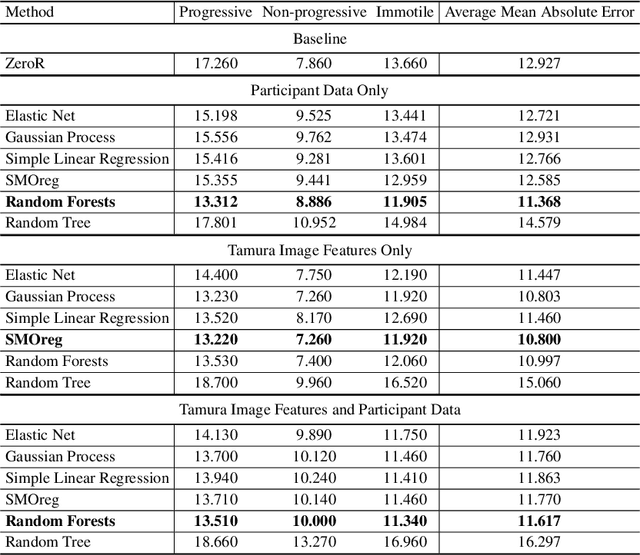
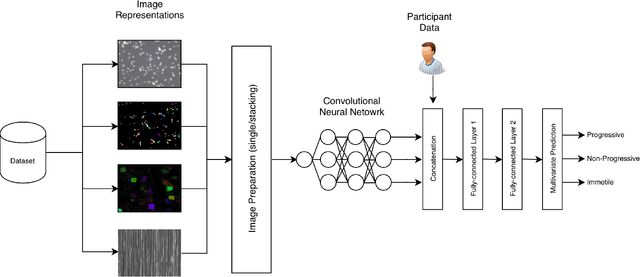
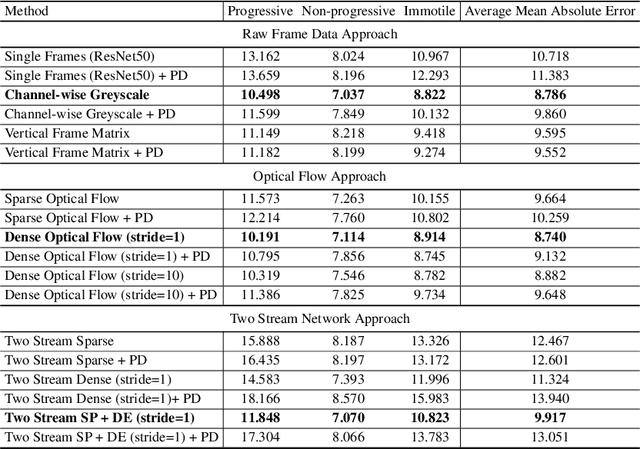
Abstract:Methods for automatic analysis of clinical data are usually targeted towards a specific modality and do not make use of all relevant data available. In the field of male human reproduction, clinical and biological data are not used to its fullest potential. Manual evaluation of a semen sample using a microscope is time-consuming and requires extensive training. Furthermore, the validity of manual semen analysis has been questioned due to limited reproducibility, and often high inter-personnel variation. The existing computer-aided sperm analyzer systems are not recommended for routine clinical use due to methodological challenges caused by the consistency of the semen sample. Thus, there is a need for an improved methodology. We use modern and classical machine learning techniques together with a dataset consisting of 85 videos of human semen samples and related participant data to automatically predict sperm motility. Used techniques include simple linear regression and more sophisticated methods using convolutional neural networks. Our results indicate that sperm motility prediction based on deep learning using sperm motility videos is rapid to perform and consistent. The algorithms performed worse when participant data was added. In conclusion, machine learning-based automatic analysis may become a valuable tool in male infertility investigation and research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge