Nghia T. Nguyen
VinDr-SpineXR: A deep learning framework for spinal lesions detection and classification from radiographs
Jun 24, 2021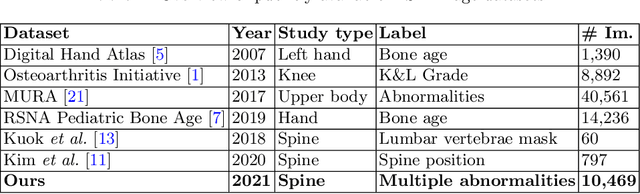
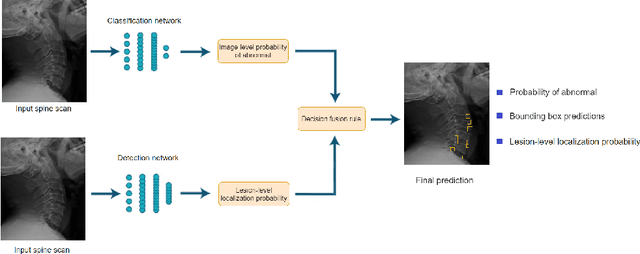
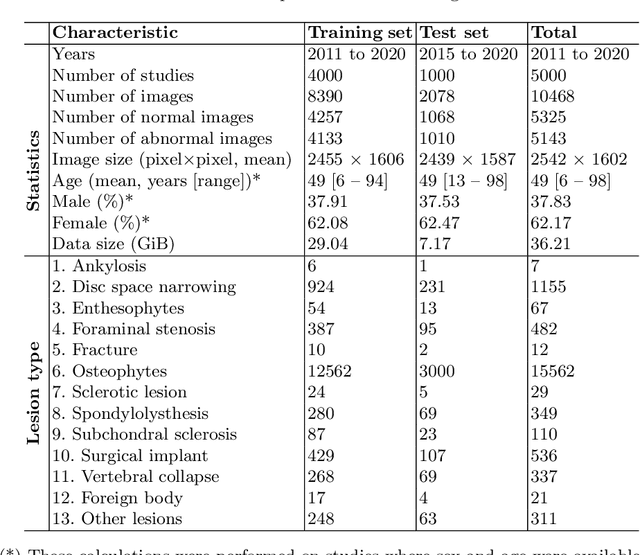
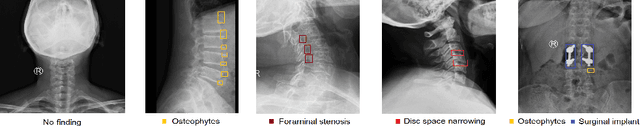
Abstract:Radiographs are used as the most important imaging tool for identifying spine anomalies in clinical practice. The evaluation of spinal bone lesions, however, is a challenging task for radiologists. This work aims at developing and evaluating a deep learning-based framework, named VinDr-SpineXR, for the classification and localization of abnormalities from spine X-rays. First, we build a large dataset, comprising 10,468 spine X-ray images from 5,000 studies, each of which is manually annotated by an experienced radiologist with bounding boxes around abnormal findings in 13 categories. Using this dataset, we then train a deep learning classifier to determine whether a spine scan is abnormal and a detector to localize 7 crucial findings amongst the total 13. The VinDr-SpineXR is evaluated on a test set of 2,078 images from 1,000 studies, which is kept separate from the training set. It demonstrates an area under the receiver operating characteristic curve (AUROC) of 88.61% (95% CI 87.19%, 90.02%) for the image-level classification task and a mean average precision (mAP@0.5) of 33.56% for the lesion-level localization task. These results serve as a proof of concept and set a baseline for future research in this direction. To encourage advances, the dataset, codes, and trained deep learning models are made publicly available.
VinDr-CXR: An open dataset of chest X-rays with radiologist's annotations
Jan 03, 2021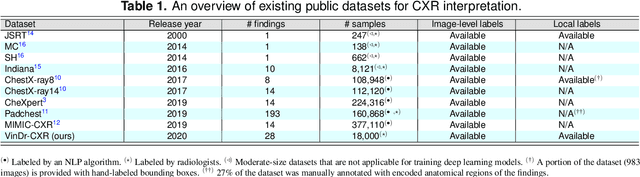
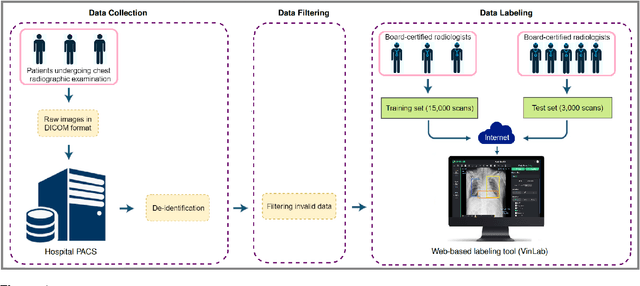
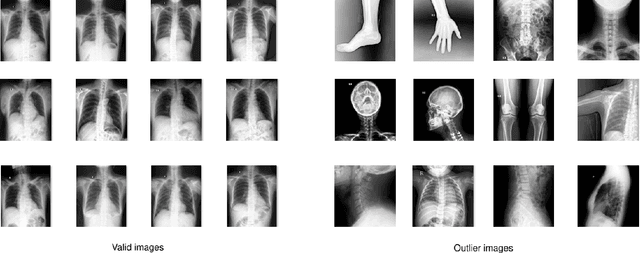
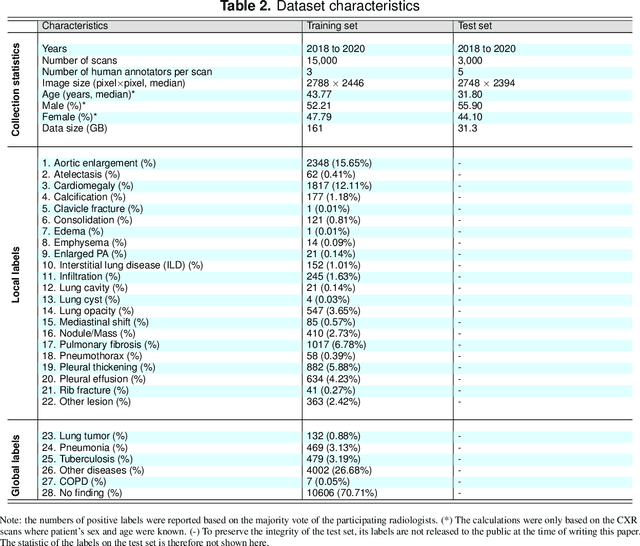
Abstract:Most of the existing chest X-ray datasets include labels from a list of findings without specifying their locations on the radiographs. This limits the development of machine learning algorithms for the detection and localization of chest abnormalities. In this work, we describe a dataset of more than 100,000 chest X-ray scans that were retrospectively collected from two major hospitals in Vietnam. Out of this raw data, we release 18,000 images that were manually annotated by a total of 17 experienced radiologists with 22 local labels of rectangles surrounding abnormalities and 6 global labels of suspected diseases. The released dataset is divided into a training set of 15,000 and a test set of 3,000. Each scan in the training set was independently labeled by 3 radiologists, while each scan in the test set was labeled by the consensus of 5 radiologists. We designed and built a labeling platform for DICOM images to facilitate these annotation procedures. All images are made publicly available in DICOM format in company with the labels of the training set. The labels of the test set are hidden at the time of writing this paper as they will be used for benchmarking machine learning algorithms on an open platform.
A CNN-LSTM Architecture for Detection of Intracranial Hemorrhage on CT scans
May 25, 2020Abstract:We propose a novel method that combines a convolutional neural network (CNN) with a long short-term memory (LSTM) mechanism for accurate prediction of intracranial hemorrhage on computed tomography (CT) scans. The CNN plays the role of a slice-wise feature extractor while the LSTM is responsible for linking the features across slices. The whole architecture is trained end-to-end with input being an RGB-like image formed by stacking 3 different viewing windows of a single slice. We validate the method on the recent RSNA Intracranial Hemorrhage Detection challenge and on the CQ500 dataset. For the RSNA challenge, our best single model achieves a weighted log loss of 0.0522 on the leaderboard, which is comparable to the top 3% performances, almost all of which make use of ensemble learning. Importantly, our method generalizes very well: the model trained on the RSNA dataset significantly outperforms the 2D model, which does not take into account the relationship between slices, on CQ500. Our codes and models is publicly avaiable at https://github.com/nhannguyen2709/RSNA.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge