Dung B. Nguyen
Slice-level Detection of Intracranial Hemorrhage on CT Using Deep Descriptors of Adjacent Slices
Aug 05, 2022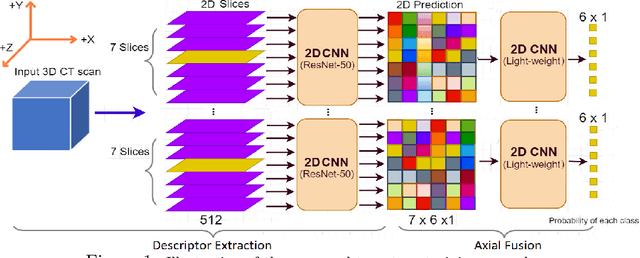
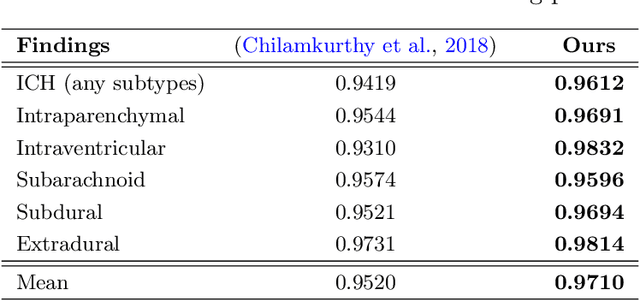
Abstract:The rapid development in representation learning techniques and the availability of large-scale medical imaging data have to a rapid increase in the use of machine learning in the 3D medical image analysis. In particular, deep convolutional neural networks (D-CNNs) have been key players and were adopted by the medical imaging community to assist clinicians and medical experts in disease diagnosis. However, training deep neural networks such as D-CNN on high-resolution 3D volumes of Computed Tomography (CT) scans for diagnostic tasks poses formidable computational challenges. This raises the need of developing deep learning-based approaches that are robust in learning representations in 2D images, instead 3D scans. In this paper, we propose a new strategy to train \emph{slice-level} classifiers on CT scans based on the descriptors of the adjacent slices along the axis. In particular, each of which is extracted through a convolutional neural network (CNN). This method is applicable to CT datasets with per-slice labels such as the RSNA Intracranial Hemorrhage (ICH) dataset, which aims to predict the presence of ICH and classify it into 5 different sub-types. We obtain a single model in the top 4\% best-performing solutions of the RSNA ICH challenge, where model ensembles are allowed. Experiments also show that the proposed method significantly outperforms the baseline model on CQ500. The proposed method is general and can be applied for other 3D medical diagnosis tasks such as MRI imaging. To encourage new advances in the field, we will make our codes and pre-trained model available upon acceptance of the paper.
A novel multi-view deep learning approach for BI-RADS and density assessment of mammograms
Dec 08, 2021



Abstract:Advanced deep learning (DL) algorithms may predict the patient's risk of developing breast cancer based on the Breast Imaging Reporting and Data System (BI-RADS) and density standards. Recent studies have suggested that the combination of multi-view analysis improved the overall breast exam classification. In this paper, we propose a novel multi-view DL approach for BI-RADS and density assessment of mammograms. The proposed approach first deploys deep convolutional networks for feature extraction on each view separately. The extracted features are then stacked and fed into a Light Gradient Boosting Machine (LightGBM) classifier to predict BI-RADS and density scores. We conduct extensive experiments on both the internal mammography dataset and the public dataset Digital Database for Screening Mammography (DDSM). The experimental results demonstrate that the proposed approach outperforms the single-view classification approach on two benchmark datasets by huge margins (5% on the internal dataset and 10% on the DDSM dataset). These results highlight the vital role of combining multi-view information to improve the performance of breast cancer risk prediction.
VinDr-CXR: An open dataset of chest X-rays with radiologist's annotations
Jan 03, 2021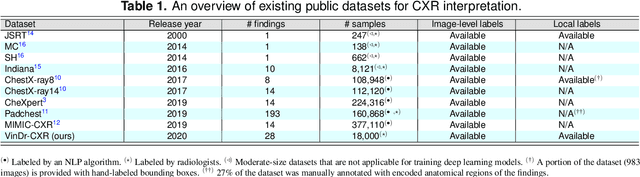
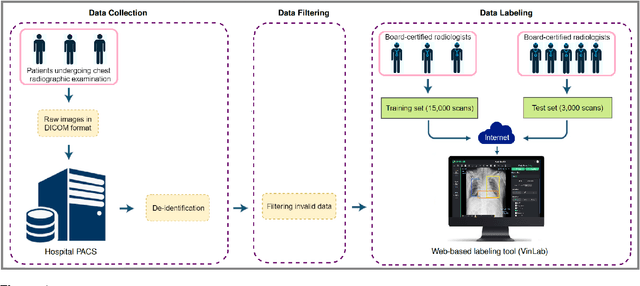
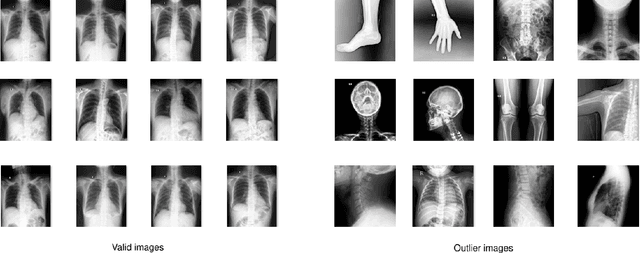
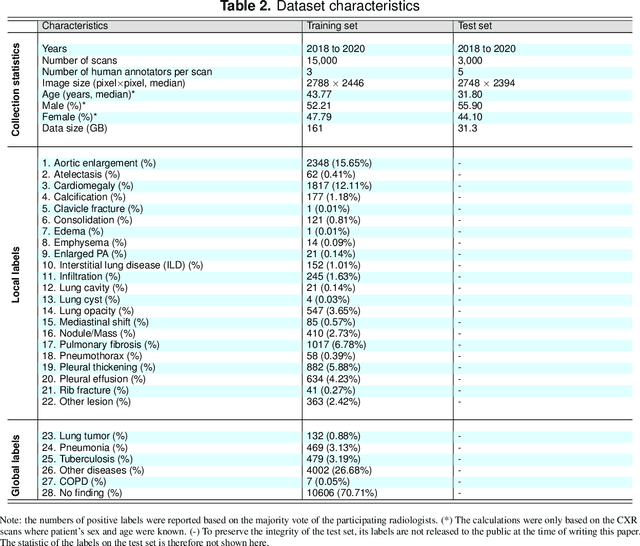
Abstract:Most of the existing chest X-ray datasets include labels from a list of findings without specifying their locations on the radiographs. This limits the development of machine learning algorithms for the detection and localization of chest abnormalities. In this work, we describe a dataset of more than 100,000 chest X-ray scans that were retrospectively collected from two major hospitals in Vietnam. Out of this raw data, we release 18,000 images that were manually annotated by a total of 17 experienced radiologists with 22 local labels of rectangles surrounding abnormalities and 6 global labels of suspected diseases. The released dataset is divided into a training set of 15,000 and a test set of 3,000. Each scan in the training set was independently labeled by 3 radiologists, while each scan in the test set was labeled by the consensus of 5 radiologists. We designed and built a labeling platform for DICOM images to facilitate these annotation procedures. All images are made publicly available in DICOM format in company with the labels of the training set. The labels of the test set are hidden at the time of writing this paper as they will be used for benchmarking machine learning algorithms on an open platform.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge