Mkael Symmonds
Department of Clinical Neurophysiology, Oxford University Hospitals, John Radcliffe Hospital, University of Oxford, UK
Multimodal wearable EEG, EMG and accelerometry measurements improve the accuracy of tonic-clonic seizure detection in-hospital
Mar 19, 2024



Abstract:Objective: Most current wearable tonic-clonic seizure (TCS) detection systems are based on extra-cerebral signals, such as electromyography (EMG) or accelerometry (ACC). Although many of these devices show good sensitivity in seizure detection, their false positive rates (FPR) are still relatively high. Wearable EEG may improve performance; however, studies investigating this remain scarce. This paper aims 1) to investigate the possibility of detecting TCSs with a behind-the-ear, two-channel wearable EEG, and 2) to evaluate the added value of wearable EEG to other non-EEG modalities in multimodal TCS detection. Method: We included 27 participants with a total of 44 TCSs from the European multicenter study SeizeIT2. The multimodal wearable detection system Sensor Dot (Byteflies) was used to measure two-channel, behind-the-ear EEG, EMG, electrocardiography (ECG), ACC and gyroscope (GYR). First, we evaluated automatic unimodal detection of TCSs, using performance metrics such as sensitivity, precision, FPR and F1-score. Secondly, we fused the different modalities and again assessed performance. Algorithm-labeled segments were then provided to a neurologist and a wearable data expert, who reviewed and annotated the true positive TCSs, and discarded false positives (FPs). Results: Wearable EEG outperformed the other modalities in unimodal TCS detection by achieving a sensitivity of 100.0% and a FPR of 10.3/24h (compared to 97.7% sensitivity and 30.9/24h FPR for EMG; 95.5% sensitivity and 13.9 FPR for ACC). The combination of wearable EEG and EMG achieved overall the most clinically useful performance in offline TCS detection with a sensitivity of 97.7%, a FPR of 0.4/24 h, a precision of 43.0%, and a F1-score of 59.7%. Subsequent visual review of the automated detections resulted in maximal sensitivity and zero FPs.
Automated Movement Detection with Dirichlet Process Mixture Models and Electromyography
Feb 15, 2023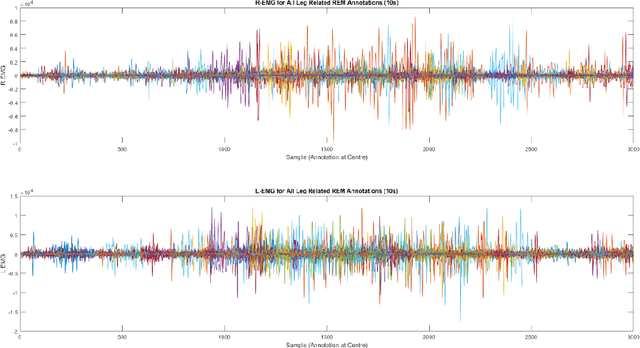
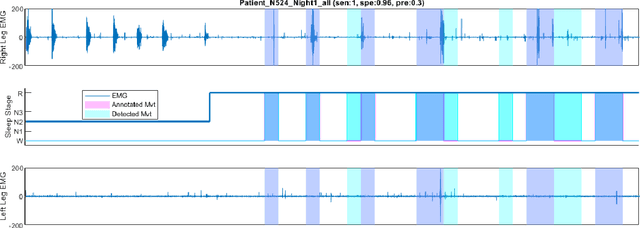

Abstract:Numerous sleep disorders are characterised by movement during sleep, these include rapid-eye movement sleep behaviour disorder (RBD) and periodic limb movement disorder. The process of diagnosing movement related sleep disorders requires laborious and time-consuming visual analysis of sleep recordings. This process involves sleep clinicians visually inspecting electromyogram (EMG) signals to identify abnormal movements. The distribution of characteristics that represent movement can be diverse and varied, ranging from brief moments of tensing to violent outbursts. This study proposes a framework for automated limb-movement detection by fusing data from two EMG sensors (from the left and right limb) through a Dirichlet process mixture model. Several features are extracted from 10 second mini-epochs, where each mini-epoch has been classified as 'leg-movement' or 'no leg-movement' based on annotations of movement from sleep clinicians. The distributions of the features from each category can be estimated accurately using Gaussian mixture models with the Dirichlet process as a prior. The available dataset includes 36 participants that have all been diagnosed with RBD. The performance of this framework was evaluated by a 10-fold cross validation scheme (participant independent). The study was compared to a random forest model and outperformed it with a mean accuracy, sensitivity, and specificity of 94\%, 48\%, and 95\%, respectively. These results demonstrate the ability of this framework to automate the detection of limb movement for the potential application of assisting clinical diagnosis and decision-making.
Detection of REM Sleep Behaviour Disorder by Automated Polysomnography Analysis
Nov 12, 2018
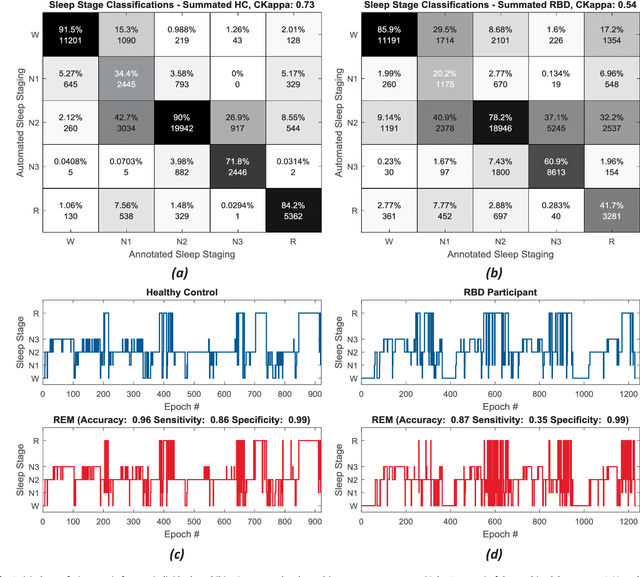
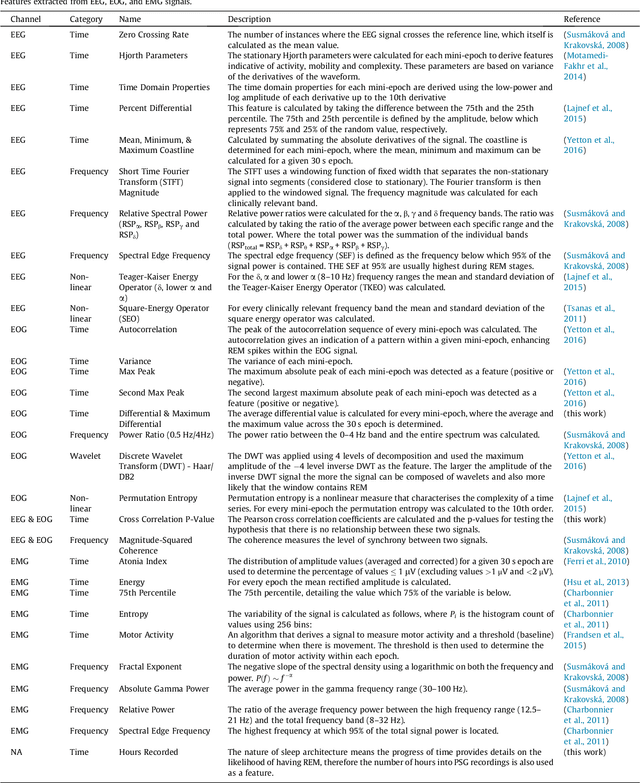
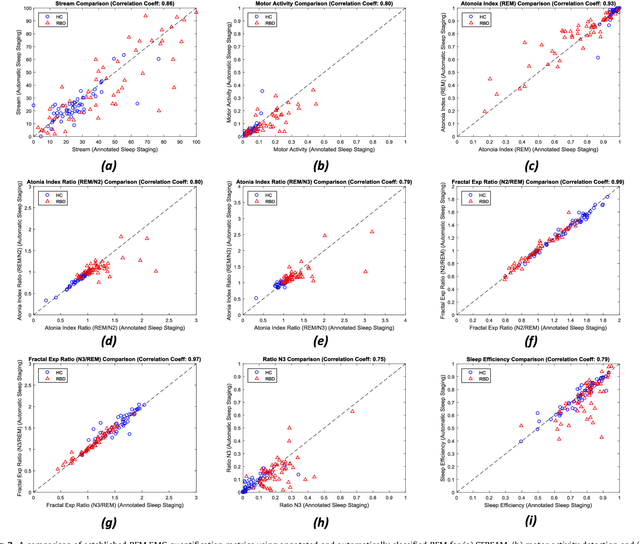
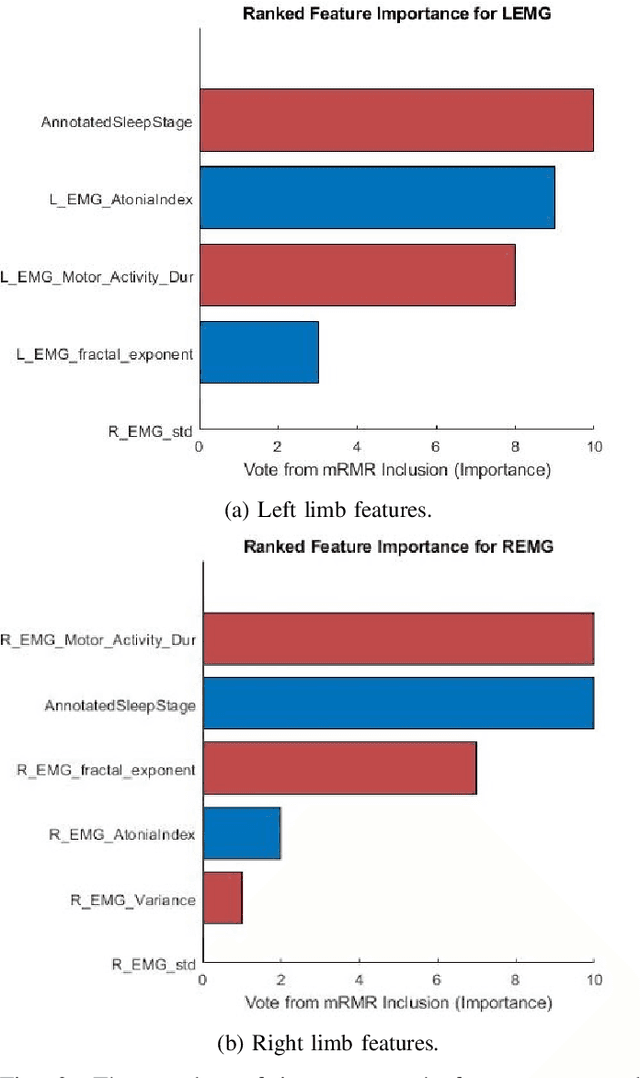
Abstract:Evidence suggests Rapid-Eye-Movement (REM) Sleep Behaviour Disorder (RBD) is an early predictor of Parkinson's disease. This study proposes a fully-automated framework for RBD detection consisting of automated sleep staging followed by RBD identification. Analysis was assessed using a limited polysomnography montage from 53 participants with RBD and 53 age-matched healthy controls. Sleep stage classification was achieved using a Random Forest (RF) classifier and 156 features extracted from electroencephalogram (EEG), electrooculogram (EOG) and electromyogram (EMG) channels. For RBD detection, a RF classifier was trained combining established techniques to quantify muscle atonia with additional features that incorporate sleep architecture and the EMG fractal exponent. Automated multi-state sleep staging achieved a 0.62 Cohen's Kappa score. RBD detection accuracy improved by 10% to 96% (compared to individual established metrics) when using manually annotated sleep staging. Accuracy remained high (92%) when using automated sleep staging. This study outperforms established metrics and demonstrates that incorporating sleep architecture and sleep stage transitions can benefit RBD detection. This study also achieved automated sleep staging with a level of accuracy comparable to manual annotation. This study validates a tractable, fully-automated, and sensitive pipeline for RBD identification that could be translated to wearable take-home technology.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge